04 Steps to Investigate Out of Specification (OOS) Result
GMPSOP
MARCH 8, 2024
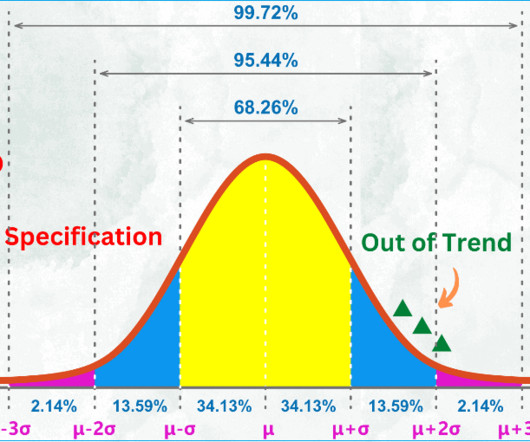
04 Steps to Investigate Out of Specification (OOS) Result Pharmaceuticals quality assurance & validation procedures GMPSOP %title% Prev PREVIOUS POST What is out of specification (OOS) result? What is the difference between OOS and OOT?









Let's personalize your content