STAT+: Drug shortages reached a record high as 2023 drew to a close
STAT
APRIL 10, 2024
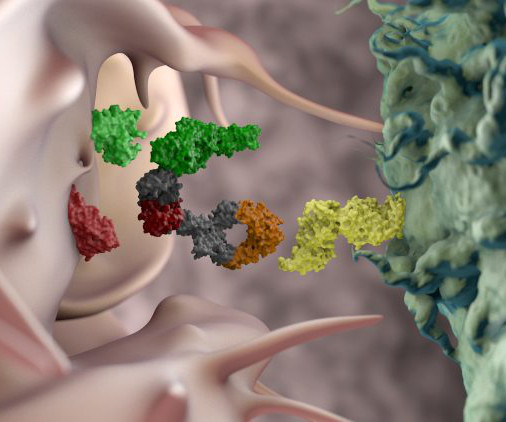
stood at 323 during the fourth quarter of last year — the highest figure reached since such data began being tracked in 2001 — underscoring growing concerns about patient harm across the country. The number of ongoing and active drug shortages in the U.S.





























Let's personalize your content