Extemporaneous Compounding
RX Note
JUNE 5, 2023
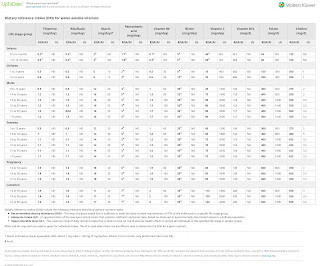
Introduction In 2018, the Ministry of Health Malaysia published the Good Compounding Practice as a guidance for compounding practices. NOTE: When compounding a preparation using the contents of an ampoule , care should be taken to withdraw the solution using a filter needle to avoid incorporating glass particles into the compound.







































Let's personalize your content