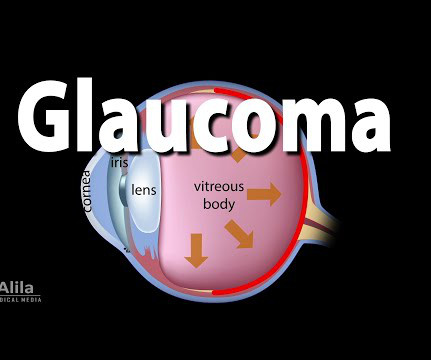
Glaucoma
RX Note
APRIL 3, 2024
External Links American Academy of Ophthalmology - Glaucoma MSD Manual Professional - Glaucoma Meta-analysis of the efficacy and safety of alpha2-adrenergic agonists, beta-adrenergic antagonists, and topical carbonic anhydrase inhibitors with prostaglandin analogs, 2010 Twelve-month, randomized, controlled trial of bimatoprost 0.01%, 0.0125%, and 0.03% (..)




































Let's personalize your content