Opinion: Compounding pharmacies offer a fix for shortages of essential drugs
STAT
MAY 15, 2023
Hospitals ran short of essential treatment medications and were unable to source those drugs from manufacturers or from the outsourcing facilities that had been authorized by Congress in 2013 to “fill the gap” in such situations.








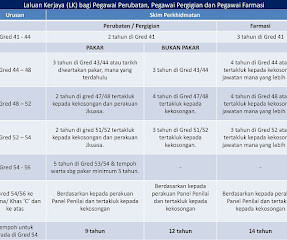
































Let's personalize your content