Dasami Lab Pvt. Ltd.-Walk-Ins for Quality Control/ Production/ Production Documentation/ EHS On 7th Jan’ 2023
Pharma Pathway
JANUARY 6, 2023
Walk-Ins for Quality Control/ Production/ Production Documentation/ EHS On 7th Jan’ 2023. is a Private incorporated on 24 July 2015. Walk-Ins for Quality Control/ Production/ Production Documentation/ EHS On 7th Jan’ 2023. Department: Quality Control/ Production/ Production Documentation/ EHS. Dasami Lab Pvt.

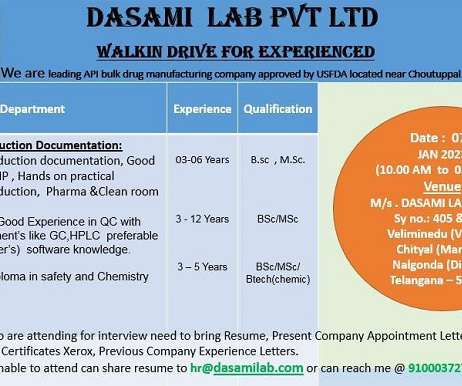





































Let's personalize your content