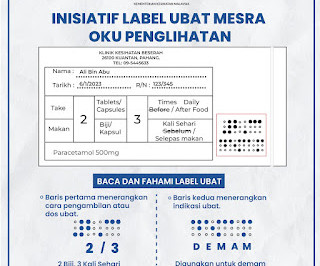
Labelling of Dispensed Medicine
RX Note
DECEMBER 25, 2024
Introduction Guide to Good Dispensing Practice, 2016 is developed to ensure that medicines are dispensed in accordance with the laws and guidelines in both government and private health care facilities in Malaysia. Labelling Requirements A ll dispensed medicine should be labelled according to the requirement stated by the law.



















Let's personalize your content