Gilead’s Vemlidy expands label to treat paediatric chronic HBV
Pharmaceutical Technology
MARCH 29, 2024
Vemlidy was first approved to treat adults with HBV in 2016. Its label was expanded in 2022 for use in patients 12 years and older.
This site uses cookies to improve your experience. By viewing our content, you are accepting the use of cookies. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country we will assume you are from the United States. View our privacy policy and terms of use.

Pharmaceutical Technology
MARCH 29, 2024
Vemlidy was first approved to treat adults with HBV in 2016. Its label was expanded in 2022 for use in patients 12 years and older.

Pharmaceutical Business Review
MARCH 29, 2024
Vemlidy, a targeted prodrug of tenofovir, was initially approved by the US regulator in 2016 for adults with chronic HBV infection and compensated liver disease. The trial results demonstrated that subjects in both the Vemlidy group and the placebo group who switched to open-label Vemlidy after 24 weeks, experienced significant improvements.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

FDA Law Blog: Biosimilars
APRIL 21, 2024
Accordingly, had Taiho marketed the product with labeling containing those errors, that labeling would have been false. Thus, regardless of the technicality of an initial notification letter stating approval on September 30, 2022, Taiho was prohibited under the FDCA from marketing LYTGOBI with false labeling, 21 U.S.C.
Eye on FDA
AUGUST 22, 2023
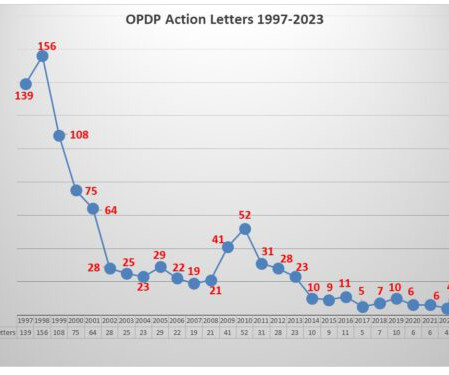
The Untitled Letter posted this week involved an oral birth control pill which has a specific of contraindications contained in the label as well as a list of warnings and precautions and of the most common adverse events. After all, back in 2016, the agency issued six letters in the month of December alone. Stay tuned.
RX Note
FEBRUARY 11, 2024
Laboratory analysis of some homeopathic remedies found greater amounts of Atropa belladonna (deadly nightshade, an anticholinergic agent) than claimed on the label. Says, 2016 FDA takes action against the use of OTC benzocaine teething products due to serious safety risk, lack of benefit, 2018
pharmaphorum
JULY 1, 2021
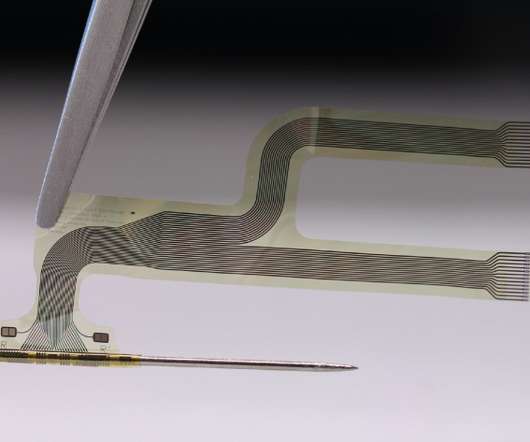
neuroloop – a spinout of Freiburg University in Germany formed in 2016 – has been working to date on using its device as an implant to lower blood pressure, among other applications. The Merck collaboration will concentrate on using the device alongside anti-inflammatory drug therapies.

pharmaphorum
AUGUST 23, 2022
The phase 1/2 trial will be an open-label, dose-escalation study that will test various doses of BV-101 in between 12 and 18 subjects. In 2019, Bayer bought cell therapy company BlueRock Therapeutics, which was created in 2016 via a joint venture between Bayer and Versant Ventures.
pharmaphorum
JUNE 8, 2022
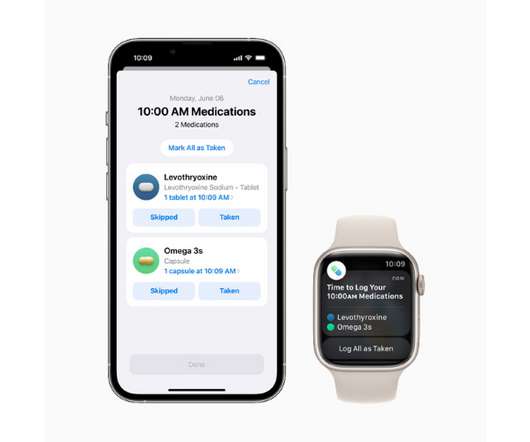
The new tool works as a component of Apple’s Health app and will let users add drugs or other health products like vitamins and supplements to a personal list – either by scanning a label or finding the product in a directory – and create custom schedules for them.

Big Molecule Watch
JANUARY 25, 2024
The FDA approved Celltrion’s infliximab biosimilar, administered by intravenous infusion, in April 2016 under the trade name INFLECTRA (infliximab-dyyb). Recommendations regarding how to describe pediatric use data in a range of scenarios and how to incorporate immunogenicity data.

Express Pharma
MAY 25, 2023
Wong adds, “ Acadia’s Nuplazid marks the first and only medication approved for the treatment of PD psychosis in the US since 2016. However, KOLs stated that Nuplazid is not very effective in managing the symptoms compared to other off-label antipsychotics.

RX Note
MARCH 18, 2023
Self-Driven Initiatives Nonetheless, multiple unofficial attempts have been made, first started with My Blue Book by My Pharmacist House (last updated to FUKKM 2016-2) , followed with Blue Book (Updated+Brand Name Search) by Apicel (last updated to FUKKM 2020-2). Similarly, the off-label uses can be clearly highlighted too.

pharmaphorum
NOVEMBER 15, 2021
Updated labelling for Invokana has already been approved to include data showing that it can reduce the risk of hospitalisation for heart failure and diabetic kidney disease in patients with type 2 diabetes, based on the CREDENCE trial. . billion in 2016 before the product was linked to an increased risk of lower limb amputation.

pharmaphorum
JUNE 6, 2022
Xalkori is billed as an ALK inhibitor and has been approved to treat ROS1-positive NSCLC since 2016, with sales of $22 million in the first quarter, but is also used to treat ALK-mutated NSCLC and lymphoma. billion product, mainly from first-line use, if it can claim FDA approval for a broad label covering any ROS1-positive cancer.

pharmaphorum
FEBRUARY 23, 2022
The drug was originally rejected for schizophrenia and bipolar by the FDA back in 2013 with a request for more clinical data, finally getting a green light two years later, and it then failed a phase 3 study in depression in 2016. Vraylar is now one of AbbVie’s fastest-growing products with sales expected to reach around $2.2

pharmaphorum
AUGUST 11, 2022
These drugs have been rigorously tested by regulatory bodies around the world before they’re made available to ensure they work as labelled, but despite that, adverse events crop up. In 2016, the estimated annual cost of drug-related morbidity and mortality resulting from non-optimised medication therapy was $528.4

RX Note
OCTOBER 5, 2023
Meanwhile, an unnecessary health risk is created in the home whereby the patient or family members may accidentally consume expired medicines or wrong drug (due to unclear or damaged labels). Failure to discard medications properly may also contaminate the environment through poor disposal either via trash or sewer system.

RX Note
JUNE 5, 2023
A simple method is to use a small adhesive label to mark the position and thus produce a measure with just one graduation.) Extemporaneous Preparations for Pediatric, Geriatric and Special Needs Patients, 2016 emphasized the need to prepare spironolactone suspension in compliance with USP 800 guidelines.

RX Note
MARCH 25, 2023
Dermatology Use (Off-label) When used in melasma , the reported success rate is up to 89%, with results appearing as early as 8 weeks. Dosage varies, according to the heaviness and duration of bleeding: 1 to 1.5 g 3 or 4 times daily for the first 3-5 days of each cycle.
pharmaphorum
JULY 12, 2021
Merck’s chief science and technology officer Laura Matz said that Innerva’s platform could become “a true enabler for digital personalised treatment of patients suffering from severe and chronic diseases such as inflammatory disorders” An open-label, proof-of-concept study looking at a vagus nerve-stimulating implant as a therapy (..)

pharmaphorum
SEPTEMBER 25, 2020
The new data – presented at this year’s ESMO meeting – reinforce the massive improvement in patient care that Keytruda has achieved in previously-untreated NSCLC, which had a five-year survival rate of just 5% before the drug was approved in 2016.
European Pharmaceutical Review
JULY 5, 2022
In the future, the potential label expansion of Oncoral into other solid cancer indications will be investigated. Gastric cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up, Gastrointestinal Cancers , 2016; 27(suppl. accessdata.fda.gov/drugsatfda_docs/label/2014/020571s048lbl.pdf [Accessed 25.01.22].

FDA Law Blog: Biosimilars
APRIL 25, 2023
Note that section 3060(a) of the 21st Century Cures Act in 2016 amended section 520 of the FD&C Act and removed certain software functions from the statutory definition of a medical device. It applies whether the software is the entire device (i.e., Software in a Medical Device, or SiMD). Loose Ends IDEs.

pharmaphorum
JUNE 20, 2022
The serotonin (5-HT2A) receptor-selective inverse agonist has been approved for Parkinson’s psychosis since 2016, and brought in $481 million from that indication last year, but remains Acadia’s only commercial product. At one point it had been tipped as a future blockbuster.
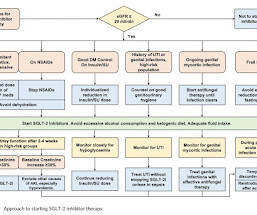
RX Note
JUNE 10, 2023
In several cases, the presentation of DKA was atypical with patients having only moderately elevated blood glucose levels, and some of them occurred during off-label use. Serious, life-threatening, and fatal cases of diabetic ketoacidosis have been reported rarely in patients taking an SGLT-2 inhibitor.

pharmaphorum
OCTOBER 27, 2020
The PolarisDMD study failed across the board, leaving Catabasis with no choice but to abandon the drug, including an ongoing open-label extension study from an earlier failed trial in the muscle-wasting disease. We are deeply saddened and disappointed by the results of our Phase 3 PolarisDMD trial,” said Jill Milne, Catabasis’ CEO. “I

pharmaphorum
OCTOBER 27, 2022
The two companies have been working together on this microbiome project since 2016. In the open-label extension study ECOSPOR IV (SERES-013), 263 adults with rCDI were evaluated when given a commercial dose of SER-109 to fulfil FDA requirements for the oral therapeutic’s safety database.

Pharmacy Joe
APRIL 20, 2022
This was a multicenter, open-label, noninferiority randomized clinical trial of 206 ICU patients with VAP. The Gram stain results were categorized as gram-positive cocci (GPC) chains, GPC clusters, gram-positive bacilli, gram-negative rods (GNR), or a combination of these.

pharmaphorum
JULY 11, 2021
The letter follows the extraordinary situation in which Biogen and partner Eisai asked the FDA to restrict the label for Aduhelm, as the agency approved the drug for all Alzheimer’s patients, not the mildly-affected population tested in clinical trials. pic.twitter.com/iWJNxdZ5Cs.

National Association of Boards of Pharmacy
AUGUST 29, 2022
In Costa Rica, pre-filled syringes labeled “Tuberculin” were among the 11,187 units of unregistered, fake, and expired medicines seized at the border. This year, regulatory authorities and officials in Northern I r eland discovered diazepam, pregabalin, inhalers, and tamoxifen among the 242,000 units of medicines seized.
RX Note
MARCH 28, 2023
To illustrate, every now and then, I may encounter an off-label indication of medication that I do not know of. Today (March 2023), you may face some difficulty in finding the GOLD 2016 Report. Nevertheless, I still miss my free lunch, and free pens much. In year 2017, GOLD began advocating for the use of LAMA or LABA.

FDA Law Blog: Biosimilars
FEBRUARY 21, 2024
This amendment marks the first significant revision of Part 820 since 1996, which established the Quality System (QS) regulation and “included requirements related to the methods used in, and the facilities and controls used for, designing, manufacturing, packaging, labeling, storing, installing, and servicing of devices intended for human use.”
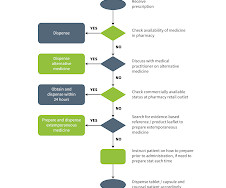
RX Note
MARCH 23, 2023
Product should be labelled clearly and stored as recommended within the formula. Pharmacy personnel are reminded not to empirically change flavourings or suspending agents because they can affect the pH and stability of the product and result in an unstable product.

FDA Law Blog: Biosimilars
JANUARY 18, 2024
FDA conducted the eight-factor scheduling analysis required by the CSA in 2016 and found that marijuana continued to meet the scheduling criteria for remaining in schedule I. 12, 2016); Denial of Petition to Initiate Proceedings to Reschedule Marijuana, 81 Fed. Denial of Petition to Initiate Proceedings to Reschedule Marijuana, 81 Fed.

Pharmacy Joe
SEPTEMBER 6, 2021
Episode 641: How Pharmacists Can Improve Their Working Relationships With Physicians (and Nurses) Subscribe on iTunes , Android , or Stitcher The idea of purposefully cultivating relationships with healthcare professionals came up when I was training a PGY-1 resident recently, and I wanted to share this episode from way back in July 2016, episode 106 (..)

The Thyroid Pharmacist
SEPTEMBER 6, 2023
Personal Care : The Wellnesse line of haircare and toothpastes by Katie Wells (aka the “Wellness Mama”) contains high-quality, safe ingredients that are clearly labeled, so there are no surprises! Published 2016 May 24. (My readers can use code IZABELLAWENTZ for 15% off all Starter Kits.) 2010;20(7):755-761. doi:10.1089/thy.2010.1636

The Thyroid Pharmacist
OCTOBER 7, 2022
Sure enough, she read all the labels on her supplements and in her pantry, and gave her kitchen a stevia-free makeover. I encourage you to read the labels and look for 100% pure, USDA-approved stevia products where possible. doi:10.1155/2016/9132052. She also stopped baking with stevia — and was able to sleep like a baby!

PharmaShots
MARCH 9, 2023
In 2016, she received a special career-recognition award from Fundamed Foundation (a non-profit Spanish foundation) in the category of the most influential pharmaceutical executive from Spain over the past 15 years Education: Belén is an alumnus of the University of Alcala de Henares as an MS, Ph.D.,

FDA Law Blog: Biosimilars
JULY 6, 2023
We now see that the proposed rule to “harmonize and modernize” the QSR with ISO13485:2016, creating the new QMSR, is on the Spring 2023 Unified Agenda (see here ). In fact, the priority designation for the final rule is labeled as “economically significant.” According to the Unified Agenda, the proposed rule is in the final rule stage.
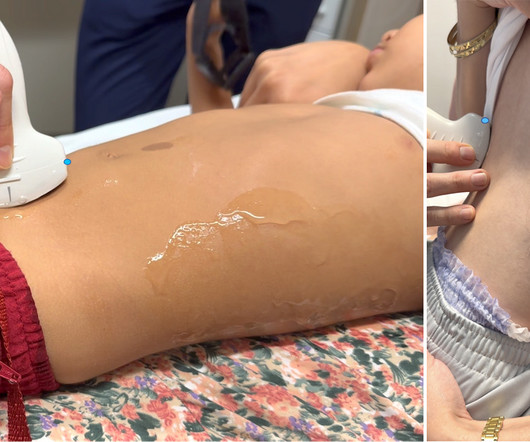
ALiEM - Pharm Pearls
MARCH 7, 2024
Longitudinal view of the right kidney: Left – Probe placement in right mid-axillary line; Right – Unlabeled and labeled ultrasound view Video 1. Acad Emerg Med , 2018 [11] Systematic review & Meta-analysis, Multicenter, 2005 Through April 2016 N=1,773, Adults POCUS has modest diagnostic accuracy in adults for nephrolithiasis.

The Thyroid Pharmacist
DECEMBER 28, 2022
When it leaks into the bloodstream, the body does not recognize it, and labels the structure as a foreign invader and attacks it. Updated March 18, 2016. However, because gluten, or more specifically the protein gliadin within gluten, mimics the structure of the thyroid, the body starts an attack on the thyroid as well. References.

RX Note
JULY 29, 2023
Off-Label Use Whenever possible, medicines for children should be prescribed within the terms of marketing authorization (product license). However, in reality, many drugs are used off-label for the neonate and paediatric population. 2021 Paediatric Formulary APK

The Thyroid Pharmacist
JUNE 30, 2023
Dr. Bernard Bihari is credited with making these discoveries about using naltrexone off-label, at lower doses. Published 2016 Sep 29. These include Crohn’s, MS, and Hashimoto’s, as well as other immune system-related conditions such as cancer and HIV/AIDS. LDN is truly an intriguing drug. I have seen people be able to get rid of pain.

ID Stewardship
MARCH 12, 2023
It’s from 2016 about the future of antibiotics and resistance, but it remains highly relevant today and people consistently give positive feedback about it. ” Labeling the use of an antibiotic as inappropriate or appropriate cannot simply be done based upon whether it is FDA-approved for a given indication.
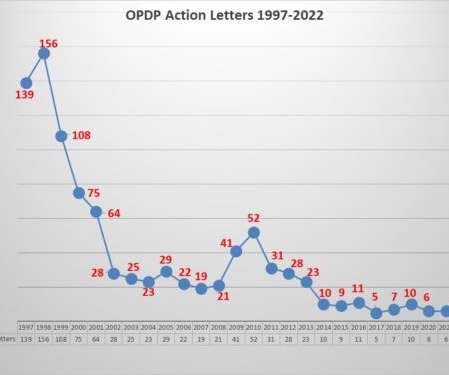
Eye on FDA
AUGUST 2, 2022
Most notably, while promotion of an unapproved drug is a relatively rare violation, comprising only 2 percent of all the letters issued since 2004, it is 13 percent of all letters issued since 2016. When you view enforcement activity through a broader lens to get more examples to analyze, a few things stand out.
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?


Let's personalize your content