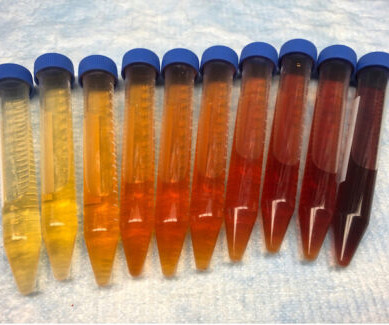
ACMT Toxicology Visual Pearl: Who Doesn’t Like a Nice Rosé?
ALiEM - Pharm Pearls
NOVEMBER 1, 2023
Clinical pharmacology, therapeutic use and potential of COMT inhibitors in Parkinson’s disease. 22 Aug 2018. Medicine (Baltimore) 1999;78(6):361-9. PMID 10575418 Kaakkola S. 2000 Jun;59(6):1233-50. PMID: 10882160 The meaning behind the color of urine. The Meaning Behind the Color of Urine – Urology Care Foundation.













Let's personalize your content