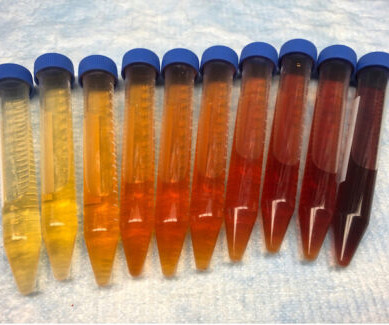
ACMT Toxicology Visual Pearl: Who Doesn’t Like a Nice Rosé?
ALiEM - Pharm Pearls
NOVEMBER 1, 2023
McGraw-Hill Education; 2019 Madiwale T, Liebelt E. Hypersensitivity reactions to rifampin: Pathogenetic mechanisms, clinical manifestations, management strategies, and review of the anaphylactic-like reactions. Clinical pharmacology, therapeutic use and potential of COMT inhibitors in Parkinson’s disease.











Let's personalize your content