Gates Foundation to fund trial of long-awaited new tuberculosis vaccine candidate
STAT
JUNE 28, 2023
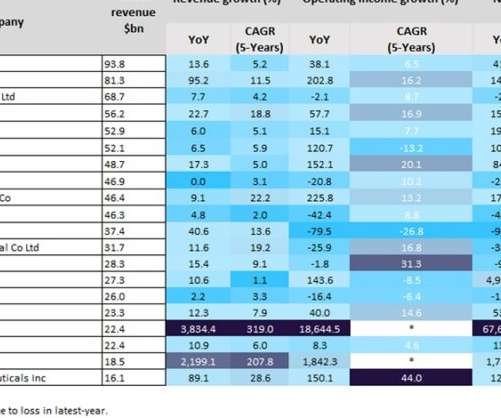
The Gates Foundation unveiled plans Wednesday to fund a long-awaited trial for what, if proven effective, would be the first new tuberculosis vaccine in over a century. The 26,000-person, Phase 3 study, set to begin next year, will test a vaccine known as M72/AS01 that showed promising results from a smaller trial in 2019.

























Let's personalize your content