Opinion: Compounding pharmacies offer a fix for shortages of essential drugs
STAT
MAY 15, 2023
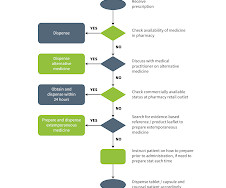
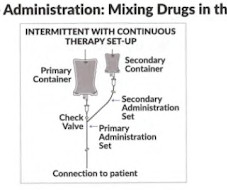
At the urging of the Alliance for Pharmacy Compounding, the trade association I lead, the Food and Drug Administration in April 2020 issued temporary guidance allowing traditional compounding pharmacies, within tight regulatory guardrails, to prepare 13 Covid drugs from pure ingredients to meet hospitals’ urgent need.




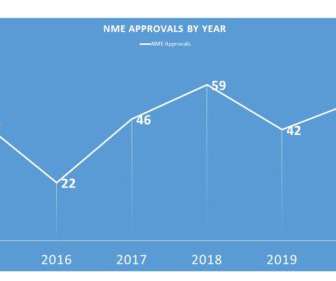
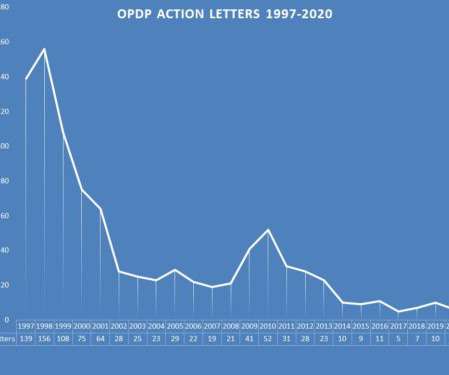

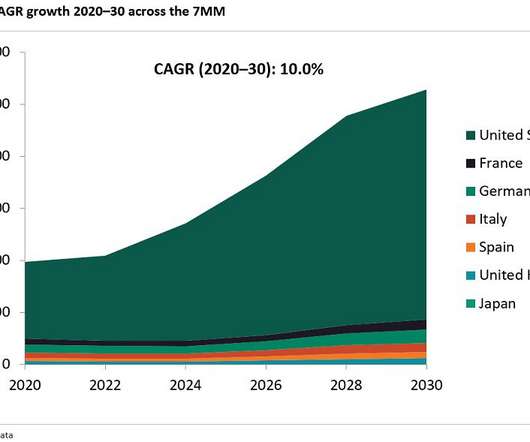

































Let's personalize your content