STAT+: Pharmalittle: We’re reading about Boehringer biosimilar frustrations, FTC warnings, and more
STAT
APRIL 5, 2024
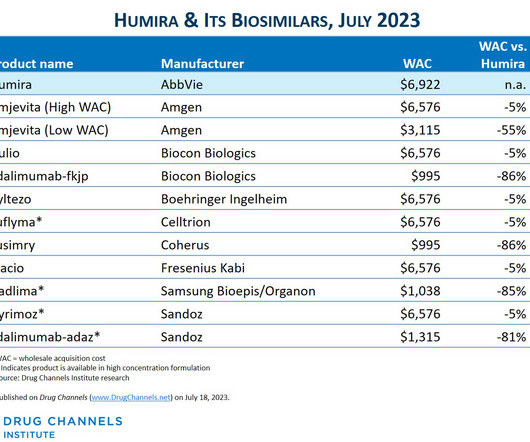
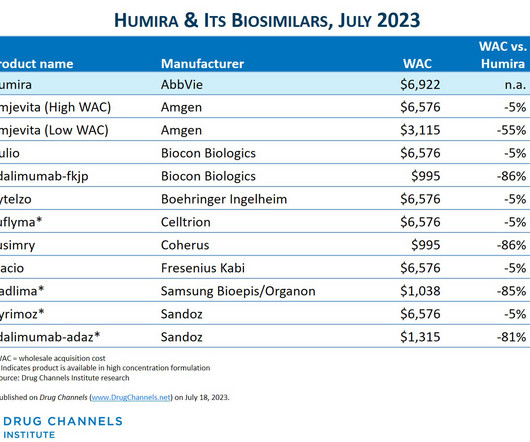
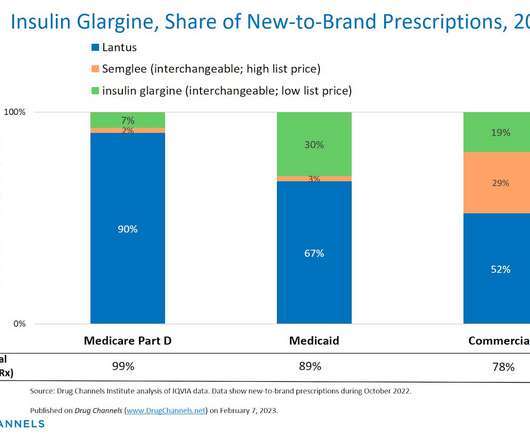
sales of its biosimilar version of AbbVie’s blockbuster arthritis treatment Humira , STAT reports. That led to less uptake of biosimilar versions of Humira in the U.S., IPV is made by only two companies — Sanofi Pasteur and Serum Institute of India, which started its supplies in 2021.








































Let's personalize your content