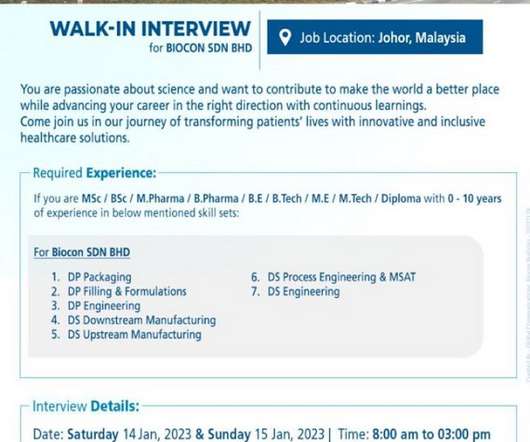
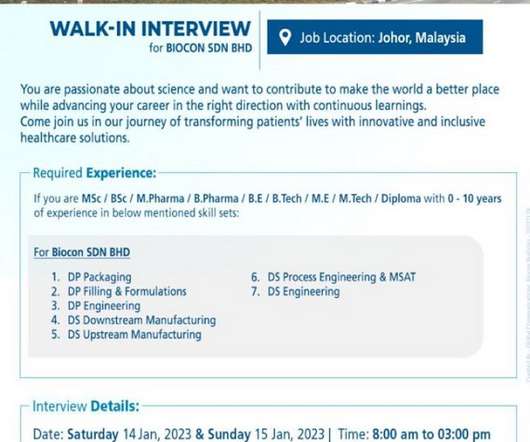
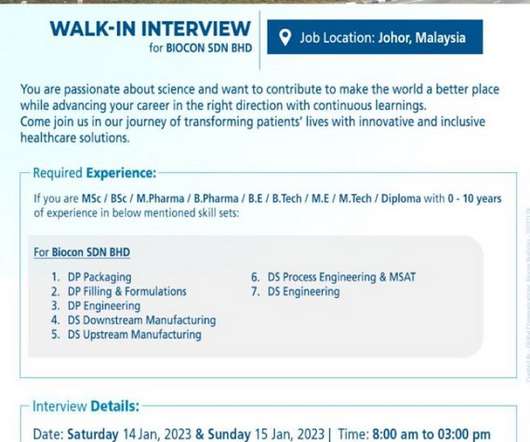
Biocon Biologics-Walk-In Drive for M.Sc/ B.Sc / M.Pharm/ B.Pharm/ BE/ B.Tech/ M.E/ M.Tech / Diploma On 11th Jan’ 2023
Pharma Pathway
JANUARY 10, 2023
M.Tech / Diploma On 11th Jan’ 2023. M.Tech / Diploma On 11th Jan’ 2023 @ Biocon Biologics. M.Tech / Diploma with 0-10 years of experience in below mentioned skill sets: For Biocon BHD: DP Packaging. Date: 11th Jan’ 2023. Required Documents: Resume. M.Tech / Diploma On 11th Jan’ 2023.



































Let's personalize your content