Preparing for an Inspection or Accreditation Survey – Part 2
National Association of Boards of Pharmacy
APRIL 10, 2024
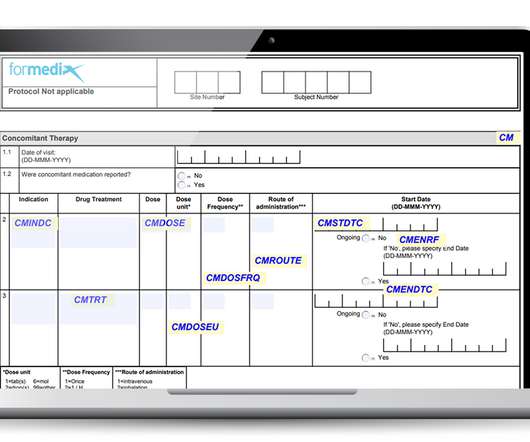
Then, walk through each step of the practice of pharmacy such as data entry; drug utilization review; compounding; final association between the drug, the prescription, and the label (eg, the dispensing act); and patient counseling.
















Let's personalize your content