Complex generics: Are global regulators addressing the needs?
Quality Matters
JUNE 26, 2023
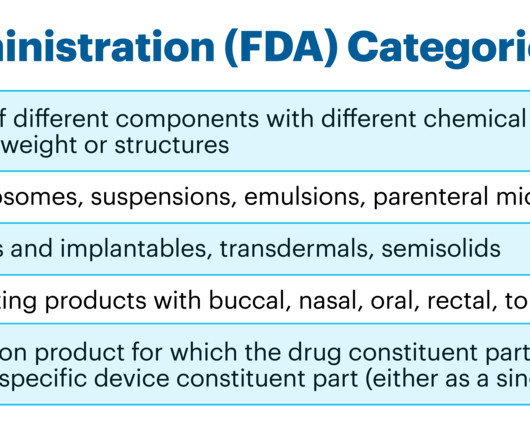
FDA 1 Even the requirements for TE for different types of products within individual NBCP categories may not be the same. FDA categories as complex products and/or of guidelines for development and approval of their generic versions. link] (accessed on June 12, 2023) 7 Regulatory harmonization. FDA and EMA. WHO Drug Inf.














Let's personalize your content