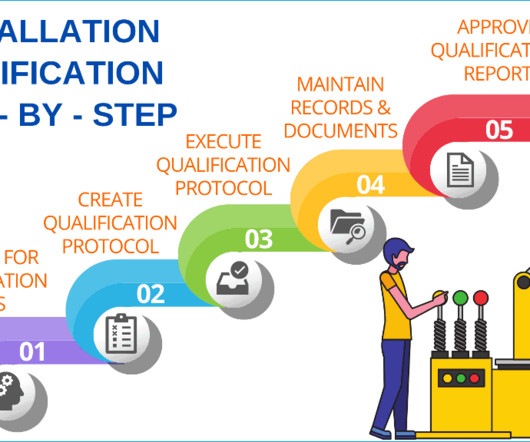
Six steps process to implement change control management
GMPSOP
MAY 26, 2023
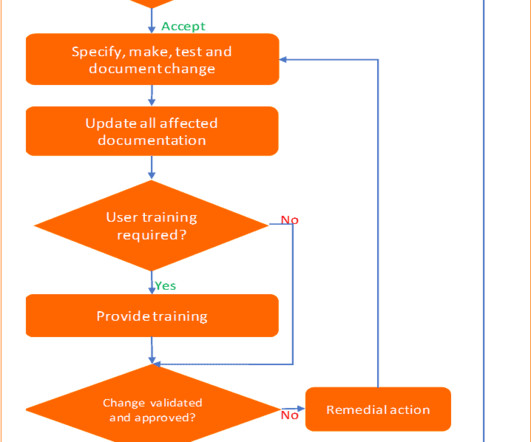
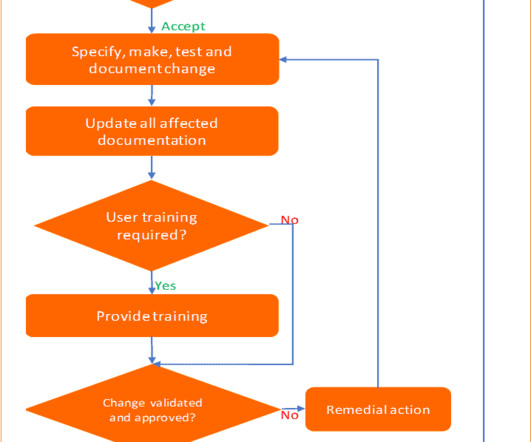
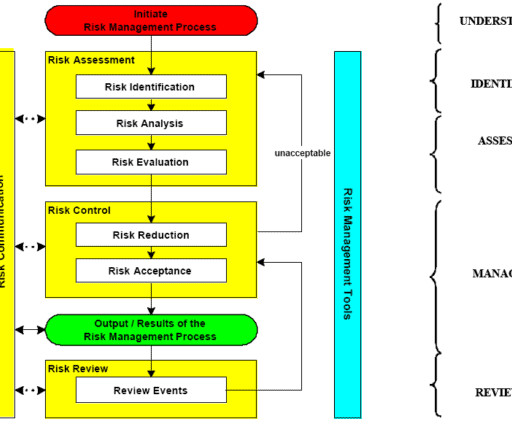
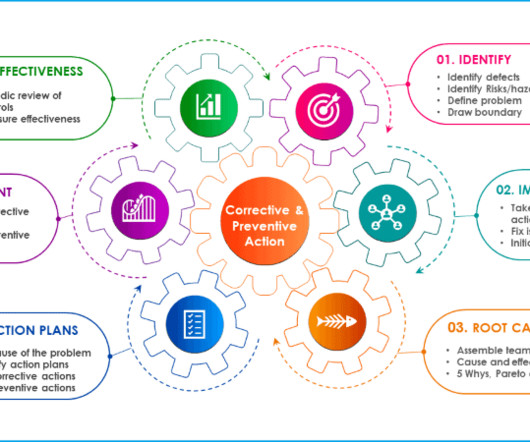
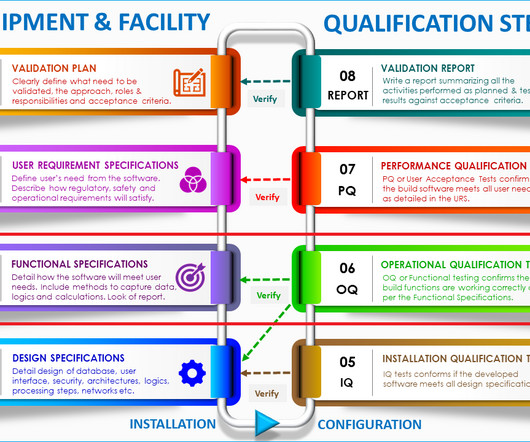
Change control management is a systematic process by which a change to facilities, products, systems, or processes is proposed, assessed by a committee (technical and operational impacts), approved, implement, reviewed for effectiveness, and communicated to a larger audience. All documentation must be kept as supporting evidence.





























Let's personalize your content