FDA-Approved Labeling: Is Enough Enough?
FDA Law Blog: Biosimilars
AUGUST 21, 2023
Livornese — I saw the sign…and the answer is no—FDA-approved labeling apparently is not enough under state failure-to-warn laws, according to certain courts.
This site uses cookies to improve your experience. By viewing our content, you are accepting the use of cookies. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country we will assume you are from the United States. View our privacy policy and terms of use.

FDA Law Blog: Biosimilars
AUGUST 21, 2023
Livornese — I saw the sign…and the answer is no—FDA-approved labeling apparently is not enough under state failure-to-warn laws, according to certain courts.
Eye on FDA
AUGUST 22, 2023
The Untitled Letter posted this week involved an oral birth control pill which has a specific of contraindications contained in the label as well as a list of warnings and precautions and of the most common adverse events. The communications vehicle in question was a social media sponsored posting.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

pharmaphorum
MAY 25, 2022
When events moved online, societies and associations recognised the wider access afforded by “virtual”, and now, as the world slowly gets back on track, many have opted for a hybrid model that offers the best of both. Identifying the appropriate code and label. The same cannot be said for online events. The codes still apply.
pharmaphorum
AUGUST 24, 2020
In the United States, the 21st Century Cures Act encouraged the Food and Drug Administration (FDA) to review and communicate patient experience data from trials – but the lack of a common framework for submissions and space on product labels has, until now, been something of a stumbling block. . Project Patient Voice .

pharmaphorum
OCTOBER 7, 2021
If there’s one take-home message about working during a pandemic, it is the enormous value that virtual events, webinars and e-learning can bring when face-to-face meetings and presentations are impossible. Rewind to March of 2020, and pharma companies were facing a communication crisis. The crisis that changed everything.

pharmaphorum
NOVEMBER 29, 2022
However, Science Magazine and STAT have reported two different patient deaths in the trial, based on not-yet-public adverse event reports and interviews with people familiar with the trial. STAT reported last month that a male patient died in June of a cerebral haemorrhage after taking the drug along with the common anticoagulant Eliquis.

pharmaphorum
OCTOBER 31, 2022
Our approach is to create a strawman schematic of the required model design based on the disease and therapeutic structure, along with a future event audit. Apply a trending and event-based forecast methodology. Then, use events to model how the market will change in the future if, for example, a new product comes to market.

pharmaphorum
MARCH 15, 2022
The vast majority of societies were able to transition their planned physical events to virtual formats within remarkably short periods of time, and some delivered impressive attendee experiences. Figure 1 What doctors say about virtual events . . And the evolution continues.

Viseven
NOVEMBER 17, 2022
Medical affairs in Pharma are often seen as a central agency that works within a healthcare company and prioritize communication among life science organizations, medical professionals, healthcare providers, and patients. Medical affairs definition uses clinical and scientific information to communicate the efficiency of a drug.

PharmaState Academy
DECEMBER 12, 2023
. ———————— Compliance in Communication: Significance and Risks NDA stressed the importance of adhering to regulations to prevent financial, reputational, and litigation costs.
Fuld & Company Blog
FEBRUARY 6, 2023
They must collect data including professional aspirations, past performance, career goals, life events, leadership and teamwork traits, motivators, consumption behavior, psychographics, and sentiments related to diversity, equality, inclusion, environment, and sustainability. These biases can creep into AI models and their recommendations.

Pharmaceutical Technology
SEPTEMBER 23, 2022
Additionally , loss of hearing at a young age is associated with impaired communication, cognitive impairment, poorer mental health and quality of life. These open-label, randomized clinical trials were done in patients under 18 years of age. Pedmark clinical trials. The therapeutic landscape.

GMPSOP
AUGUST 28, 2023
Raw materials must be inspected to confirm that the containers are intact, have been provided according to the paperwork, and have labels affixed on them identifying the raw material name, batch number, and expiry date. For example, materials with Hold, Quarantine, or Rejected labels must be kept in a quarantine location.
GMPSOP
AUGUST 23, 2022
The tool can also be used for any other quality and compliance issues where a risk event is repetitive and a quality decision is urgent. Is this system used in the creation or verification of product labelling (inserts, outserts, cartons or packaging)? process change, product recall, labelling change, adverse event/safety reporting)?

Quality Matters
AUGUST 3, 2023
ampules, bottles, labels, cartons, shipping containers, desiccants) Services (e.g., This risk assessment effort is not a one-time event but rather a periodic, recurring process for communicating and reviewing risks,” noted USP’s Horacio Pappa, Ph.D., active ingredients, excipients, other raw materials) Packaging materials (e.g.,

ID Stewardship
JUNE 6, 2023
Streamlined Prescription Filling : AI systems can automate prescription filling processes by extracting relevant information from electronic prescriptions, verifying insurance coverage, and generating labels. AI can assist in language translation, improving communication with non-native speakers.

FDA Law Blog: Biosimilars
OCTOBER 10, 2023
Labeling and Implant ID Card Lastly, it is critical that patients are provided with implant information as it pertains to their devices. separate patient labeling). FDA also recommends the implant ID card provide contact information in the event of malfunctions or adverse events.

The Honest Apothecary
AUGUST 12, 2022
lt means that acknowledging a special event in someone’s life (a birthday, marriage, accomplishment) is more important than submitting my end of month financial paperwork on time. But it tends to communicate that you don’t trust your manager if you are regularly meeting with his/her direct reports with an agenda of your own.

pharmaphorum
SEPTEMBER 15, 2020
The huge rise in the use of telemedicine services during lockdown has brought care directly to patients in their homes, while pharma’s communications with healthcare professionals (HCPs) has experienced a similar push to digital channels. The mindset that accomplished this needs to be retained,” Jeremy says. About the interviewees.
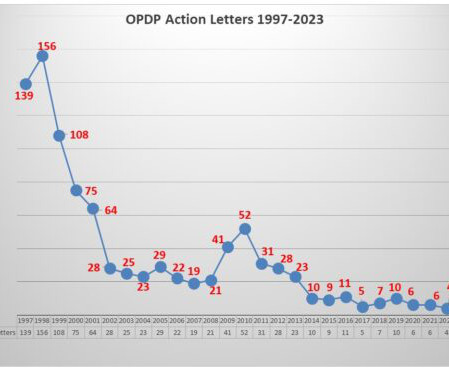
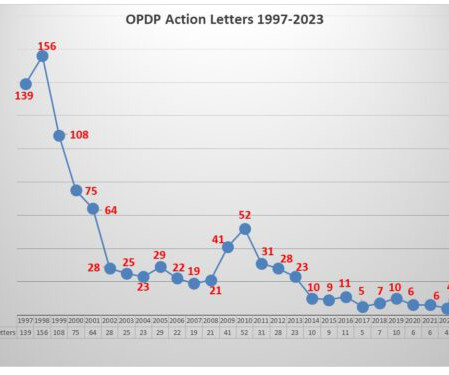
Eye on FDA
JUNE 14, 2023
The label for the medicine includes a boxed warning regarding potential issues related to complications for the liver (toxicity) and heart (QT prolongation). Going back and looking through 2020 at the products involved in letters, there have been a total of 17 letters (involving 19 different communications vehicles).
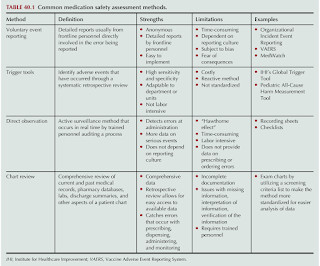
RX Note
OCTOBER 5, 2023
Medication Error The term "medication error" is used to describe any preventable event that may cause or lead to inappropriate medication use or patient harm while the medication is in the control of the healthcare professional, patient or consumer. Use Tall Man lettering Use additional warning labels for LASA medicines.
GMPSOP
MARCH 17, 2023
Immediately communicate the incident to stakeholders and initiate an internal laboratory investigation. – Status control: implementing status labels to clearly identify workflow status such as issues, returns, rejects and reworks of components to prevent mix ups. No, this will be a violation of good laboratory practice.

European Pharmaceutical Review
JANUARY 23, 2023
This has been exacerbated by geopolitical events such as war, the energy crisis and high inflation rates. MSSG stresses the importance of transparency in relation to shortages and highlights the need for all stakeholders to communicate in an objective and responsible way to avoid any undue public concern.
RX Note
DECEMBER 16, 2023
Introduction Medication error is any preventable event that may harm patient. This incident highlights the potential dangers of unconventional medical abbreviations in prescription communication. I did not identify any errors on medication label and the quantity prepared. The prescription was a simple one: T.
GMPSOP
MARCH 4, 2023
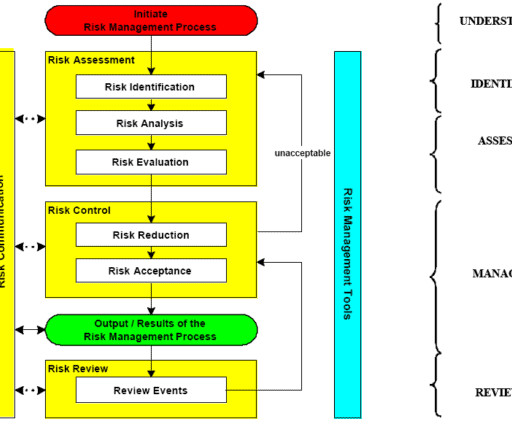
Quality risk management involves the art and science of identifying, analyzing, assessing, and managing uncertain events. Events that can impact product quality or compliance with registered dossier throughout a product’s life. In most cases, these questions need to be asked before a risk event would potentially occur.

Digital Pharmacist
OCTOBER 11, 2023
These events can cover various topics, such as understanding prescription labels, proper medication use, managing chronic conditions, and reading nutritional labels. You can host a workshop or seminar at your pharmacy location or at a restaurant in your community.

Pharma Marketing Network
JUNE 11, 2020
We may do some things like you were talking about where we have advertorials, and it’s clearly labeled as an advertorial. So whether you’re an advertiser or an event coordinator or a supplier in the industry, most of them have pretty active blogs. We want to be a resource for them. I mean, everybody is a publisher.

Pharmaceutical Technology
DECEMBER 16, 2022
This submission comprises preliminary findings from a Phase II open-label, single-arm, single-dose, multicentre clinical trial of CNCT19. Cytokine release syndrome and neurotoxicity were reported to be the most prevalent adverse events (AEs) linked to CNCT19. In the trial, 82.1%
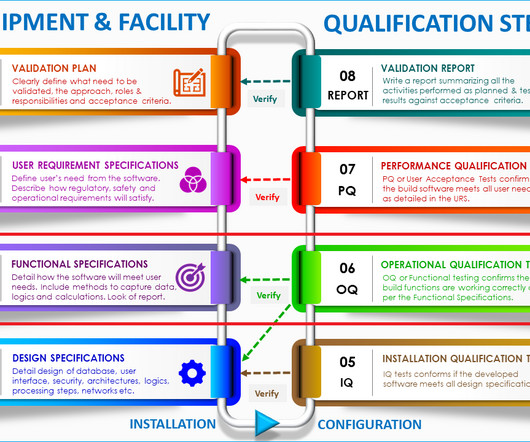
GMPSOP
JULY 31, 2023
Alarm and safety test: Acceptance Criteria: The autoclave’s alarms should activate and safety interlocks should function correctly when triggered by predefined events or conditions. 90% to 110% of the label claim). 5% of the average force).

pharmaphorum
OCTOBER 4, 2022
The FDA explains that regardless of the complexity of the software and whether or not the software is proprietary, the output or labelling should provide HCP users with adequate background information in plain language on the input(s), algorithm logic or methods, datasets, and validation.

The Thyroid Pharmacist
JANUARY 6, 2023
It breaks my heart to think about the many thyroid patients who get labeled as clinically depressed instead of being tested for thyroid antibodies, and to think of the patients who start on thyroid hormones without consideration of dosage and form, resulting in ongoing symptoms, including depression. I assure you, you can get past them.
pharmaphorum
JANUARY 8, 2021
I DID NOT consent to receive an off-label drug with NO evidence of benefit with a single dose. The NHS Confederation, which represents leaders across the organisation, told pharmaphorum the government needed to be very clear in its communications with the public about exactly what they are being asked to do and why. “We
pharmaphorum
JANUARY 8, 2021
I DID NOT consent to receive an off-label drug with NO evidence of benefit with a single dose. Just received this email cancelling my 2nd dose of the Pfizer vaccine. On the basis of UK government guidance yesterday. This means that the vaccine is not being delivered as licensed. pic.twitter.com/ZDtIjm1z8W.

The Thyroid Pharmacist
OCTOBER 27, 2023
These signals create communication and function within nerves and muscles, as the electrolytes move in or out of cells. This can be used in the event of food poisoning, accidental ingestion of a food you’re sensitive to, or for binding mycotoxins and other pathogenic gut infections. It helps eliminate unwanted microbes and toxins.

PharmaShots
APRIL 3, 2023
events/100 patient-yrs.) The results also showed a reduction in risk of hospitalization for any cause with 14% relative risk reduction (24.8

RX Note
FEBRUARY 23, 2023
G6PD Deficiency - Explaining G6PD Deficiency Ramadan - Medications during Ramadan fasting Adverse Drug Reactions Adverse Drug Reactions - All adverse reactions are adverse drug event Pharmacovigilance - To detect unreported ADRs Drug Allergy - Management vary based on the signs and symptoms Medicine-induced Discolouration - Red?

Pharma Marketing Network
OCTOBER 29, 2020
What does it mean to you to have consumer-centric or consumer centricity in your marketing communications? RJ (PMN): So so true. Often the consumers comes up as the biggest driver.

Pharmaceutical Technology
APRIL 11, 2023
This label expansion makes Orkambi the only disease-modifying CF medication available to patients of this age in Canada. This label expansion follows a similar label change in the US in September 2022. Health Canada is granting this new label expansion based on recent results from a Phase III study.

Eye on FDA
JANUARY 11, 2021
In late March FDA began issuing Coronavirus Updates labeled as a “Daily Roundup” except they weren’t actually always a daily release – some days got skipped. One additional note on FDA’s communications this past year. In all there were 165 of them. The Daily COVID-19 updates were not translated.

The Thyroid Pharmacist
OCTOBER 6, 2023
Check food labels carefully, and be extra careful if you are eating out, as it’s difficult to know all of the ingredients used in restaurants. a genetic predisposition, and a triggering event. [27] It may be best to avoid eating out during this elimination period, to avoid any cross-contamination that might skew your results.

Pharmaceutical Technology
NOVEMBER 30, 2022
weight reduction in obese individuals, as per its label. Furthermore, in order to make up for shortages Novo Nordisk’s Ozempic, a version of semaglutide that is approved to treat type 2 diabetes, was used off-label for obese patients. In a Phase II study, CagriSema achieved a numerically higher body weight reduction of 15.6%
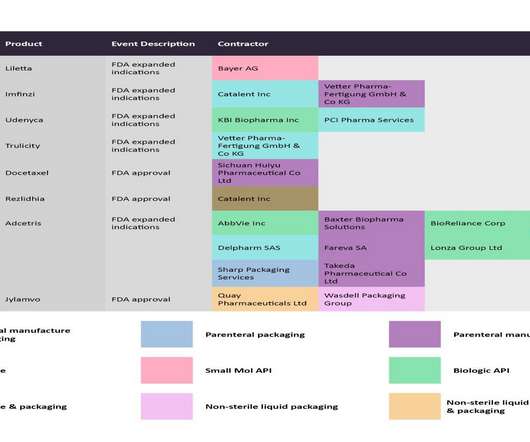
Pharmaceutical Technology
JANUARY 15, 2023
Using the manufacturing contract-related data collated from PharmSource reports, and news from the GlobalData Pharma Intelligence Center, Pharmaceutical Technology has collated some of the key regulatory events from late November to late December that will likely impact manufacturing volumes, and stakeholders that will likely be affected.

Pharmaceutical Technology
NOVEMBER 30, 2022
weight reduction in obese individuals, as per its label. Furthermore, in order to make up for shortages Novo Nordisk’s Ozempic, a version of semaglutide that is approved to treat type 2 diabetes, was used off-label for obese patients. In a Phase II study, CagriSema achieved a numerically higher body weight reduction of 15.6%

Pharmaceutical Technology
JUNE 16, 2023
Merck ( MSD ) has announced updated results with Keytruda (pembrolizumab) in the Phase III Keynote-811 trial, which opens up the possibility of changing the checkpoint inhibitor’s label in HER2-positive gastric or GEJ adenocarcinoma so it’s based on the tumour’s PD-L1 biomarker status. It showed promising event-free survival (EFS) of 62.4%
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?


Let's personalize your content