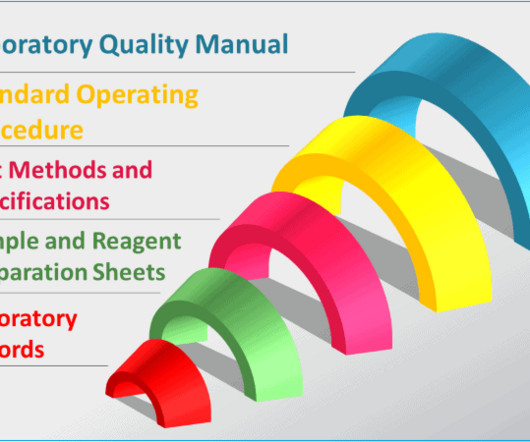
Typical GMP documentation in a quality control laboratory
GMPSOP
APRIL 3, 2023
Data management policy : This policy governs activities for data entry, storage, and retrieval to ensure the integrity of laboratory results. Record keeping policy : this policy outlines procedures for documenting laboratory activities, including data, results, and observations.








Let's personalize your content