Overview of GLP requirements on everyday laboratory operations
GMPSOP
DECEMBER 24, 2023
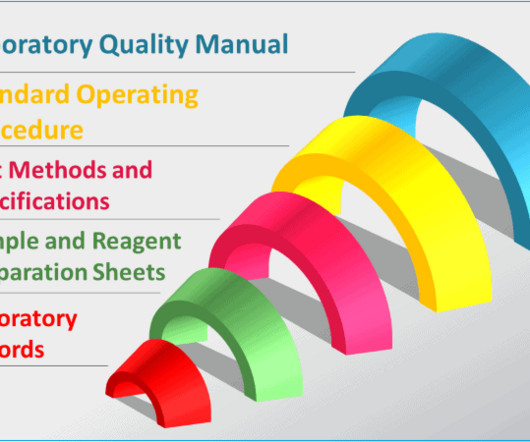
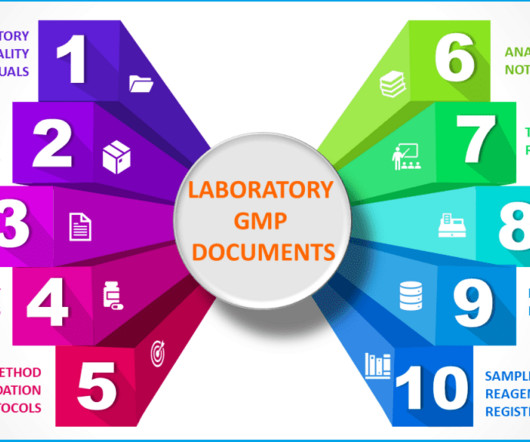
– Record or capture all generated raw data directly, promptly and legibly. – Use traceable data sheets or sequentially numbered notebooks. – Date and sign or initial data entries on the day of entry. – Test method validation protocols, data and reports – Other records and data i.








Let's personalize your content