STAT+: Day One drug for common childhood brain tumor approved by FDA
STAT
APRIL 23, 2024
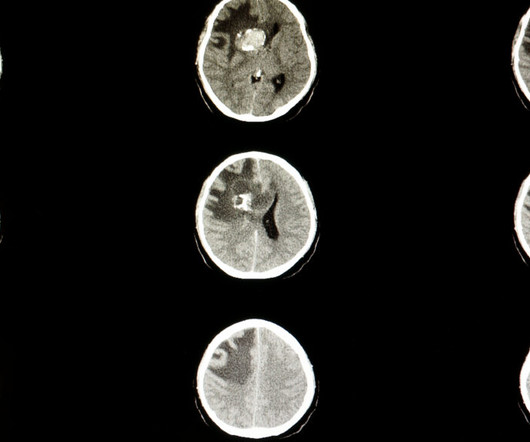
D ay One Biopharmaceuticals said Tuesday the Food and Drug Administration approved its pill for one of the most common forms of childhood brain tumors, called pediatric low-grade glioma. The drug, previously known as tovorafenib, will be marketed as Ojemda. Day One has not yet disclosed a price.






































Let's personalize your content