How to conduct product quality review in pharmaceutical
GMPSOP
NOVEMBER 19, 2023
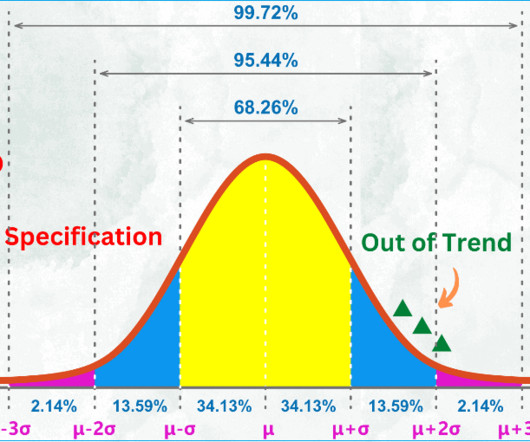
Additional documents included each month. If significant trends are identified, they should be documented, and changes should be made to avoid out-of-specification results. The number of batches manufactured, released and rejected should be documented. 240 SOPs, 197 GMP Manuals, 64 Templates, 30 Training modules, 167 Forms.





























Let's personalize your content