Leading freeze-drying systems suppliers for the pharmaceutical industry
Pharmaceutical Technology
MARCH 10, 2023
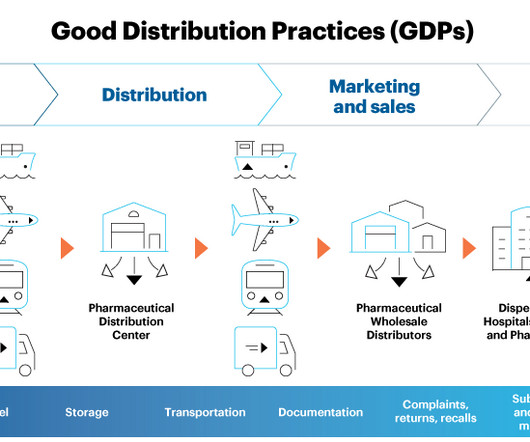
Pharmaceutical and biotechnology industries widely use freeze-drying systems to protect vaccines, antibodies, antibiotics such as penicillin, blood plasma, proteins, enzymes, hormones, viruses, and bacteria from heat and minimise their biological activity. The process eliminates the disadvantages of conventional drying methods or freezing.



















Let's personalize your content