Cleaning validation protocol for pharmaceutical industry
GMPSOP
SEPTEMBER 27, 2023
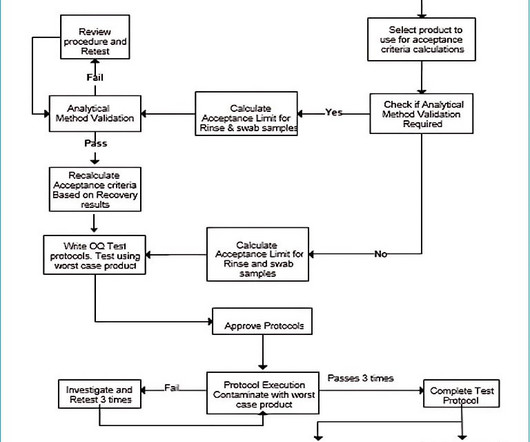
Select the worst-case product for cleaning validation For multi-product equipment, it is not practical to validate the cleaning of all products that have one cleaning process and where products are alike in formulation and dosage form. If it is not, analytical method validation is required.








Let's personalize your content