New USP Guidelines: What Changes Impact My Veterinarian Practice?
epicur
JANUARY 18, 2024
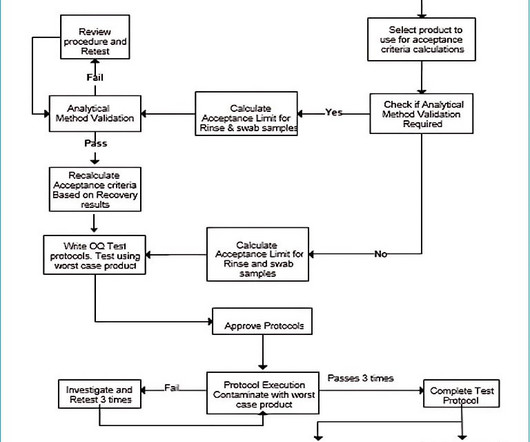
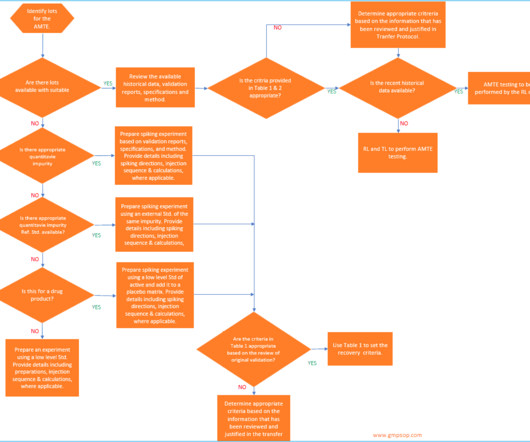
While both BUDs and expiration dates serve a similar purpose of decreasing patient risks associated with any changes a medication may undergo during storage, expiration dates have key advantages : Expiration dates have a high degree of accuracy—they are determined using stability testing methods validated by cGMP regulations enforced by the FDA.










Let's personalize your content