ESMO: Roche’s Alecensa claims a first in ALK-positive NSCLC
pharmaphorum
OCTOBER 22, 2023
ESMO: Roche’s Alecensa claims a first in ALK-positive NSCLC Phil.

pharmaphorum
OCTOBER 22, 2023
ESMO: Roche’s Alecensa claims a first in ALK-positive NSCLC Phil.

Drug Patent Watch
OCTOBER 22, 2023
Sugammadex sodium is the generic ingredient in two branded drugs marketed by Msd Sub Merck, Aspiro, Mylan, and Sandoz and, and is included in four NDAs. There is one patent… The post New tentative approval for Sandoz drug sugammadex sodium appeared first on DrugPatentWatch - Make Better Decisions.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

PharmaTech
OCTOBER 22, 2023
According to Grandview Research, the hybrid and decentralized clinical trial (DCT) market will be worth more than 12 billion dollars by 2030. With pharma having discovered the pitfalls of individual solutions, many are building a new clinical trial technology foundation that can better scale according to their needs. Hear from four top minds across pharma and discover what the next model of clinical conduct looks like, what it does for sites and patients, and why foundational platforms are criti
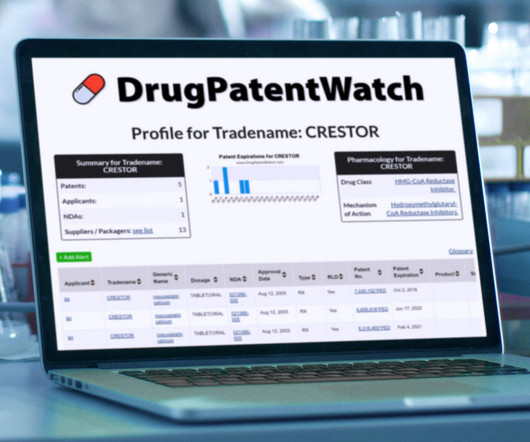
Drug Patent Watch
OCTOBER 22, 2023
Sugammadex sodium is the generic ingredient in two branded drugs marketed by Msd Sub Merck, Aspiro, Mylan, and Sandoz and, and is included in four NDAs. There is one patent… The post New tentative approval for MYLAN drug sugammadex sodium appeared first on DrugPatentWatch - Make Better Decisions.

Speaker: Chris Antypas and Josh Halladay
Access to limited distribution drugs and payer contracts are key to pharmacy expansion. But how do you prepare your operations to take the next step? Meaningful data: Collect and share clinical data regarding outcomes, utilization, and more Reporting: Limited distribution models require efficient tracking and reporting systems Workflows: Align workflows with specific pharma and payer contractual requirements For in-depth, expert insights on pharmacy expansion, watch this webinar from Inovalon.

Pharmacy Joe
OCTOBER 22, 2023
In this episode, I’ll discuss an article about cefepime vs piperacillin-tazobactam in adults hospitalized with acute infection. Episode 863: Does This Randomized Trial Settle the Increased AKI from Piperacillin-Tazobactam Question? Subscribe on iTunes , Android , or Stitcher Because piperacillin-tazobactam and cefepime have similar cost and coverage vs gram-negative bacteria, the choice of which agent to use in hospitalized patients with acute infection comes down to differences in adverse

PharmaTech
OCTOBER 22, 2023
Various factors must be considered when it comes to developing and delivering high-quality pharmaceutical drug products or medical devices to end users. A CDMO can help ensure products maintain quality standards and are readily available as necessitated throughout development, manufacturing, and commercialization.
Pharmacist Digest brings together the best content for pharmacists from the widest variety of industry thought leaders.

Pharma in Brief
OCTOBER 22, 2023
The Federal Court has upheld a decision of the Minister of Health ( Minister ) that the new owner of a biosimilar new drug submission ( NDS ) could adopt the notice of allegation ( NOA ) served by its predecessor. Consequently, the new owner was properly added as a defendant to an on-going action under subsection 6(1) of the Patented Medicines (Notice of Compliance) Regulations ( Regulations ).
Med Ed 101
OCTOBER 22, 2023
Vitamin B12 is a critical nutrient that helps with the function of major body systems. It is involved in the production of red blood cells, is important in energy production, and helps maintain a normally functioning nervous system. Vitamin B12 deficiency can lead to anemia, fatigue, weakness, numbness, tingling, and cognitive impairments. Permanent nerve damage […] The post Drugs That Cause Vitamin B12 Deficiency – Top 5 appeared first on Med Ed 101.

PharmaTech
OCTOBER 22, 2023
There are many reasons behind the success of a technology transfer. But at the center of this complexity is one critical role: project management in the tech transfer.

pharmaphorum
OCTOBER 22, 2023
ESMO: Opdivo chases Keytruda in perioperative lung cancer Phil.

Advertisement
Are you still using workarounds to manage your daily operations? To achieve peak performance, it's time to explore other options for specialty and infusion pharmacy software. Streamline pharmacy operations and improve clinical performance with automated processing, real-time data exchange, and electronic decision support. Download this helpful infographic to: Drive efficiency and patient adherence from referral receipt to delivery and ongoing care – all with our Pharmacy Cloud.

PharmaTech
OCTOBER 22, 2023
Process equipment used in the healthcare and pharmaceutical industries follow rigid specifications for accuracy, consistency and cleanliness.
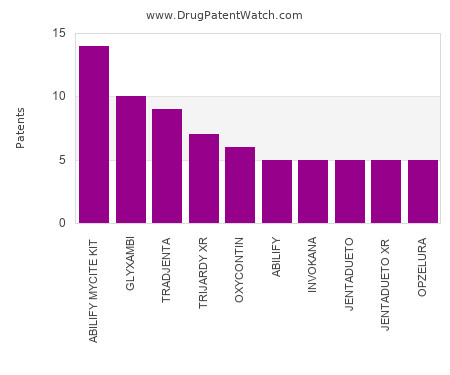
Drug Patent Watch
OCTOBER 22, 2023
This chart shows the drugs with the most patents in Malaysia. Patents must be filed in each country (or, in some cases regional patent office) where patent protection is desired.… The post Which pharmaceutical drugs have the most drug patents in Malaysia? appeared first on DrugPatentWatch - Make Better Decisions.

PharmaTech
OCTOBER 22, 2023
According to Grandview Research, the hybrid and decentralized clinical trial (DCT) market will be worth more than 12 billion dollars by 2030. In this free guide, we delve into the exciting world of DCTs and explore how they are reshaping the landscape of medical research. As technology advances and the need for more efficient and patient-centric approaches to clinical trials grows, DCTs have emerged as a powerful solution.

The Guardian - Pharmaceutical Industry
OCTOBER 22, 2023
Fantastic Beasts director David Yates’s true-life big pharma scandal is carried by the British actor playing a money-hungry drug rep David Yates takes time out from being the in-house director of the Fantastic Beasts films with a formulaic, factually based drama on a subject that has already been thoroughly explored by other, more serious-minded movies, documentaries and television series: America’s opioid crisis and the cynical workings of big pharma.

PharmaTech
OCTOBER 22, 2023
Over the last decade the number of oncology trials has skyrocketed, almost doubling the number of all other therapeutic areas combined, according to the WIRB-Copernicus Group¹. Known for their complex design, oncology trials often present various participant, site, and sponsor hurdles. Sponsors and CROs looking to tackle these challenges and reduce the burden on participants and sites should explore the potential of digital solutions, particularly electronic informed consent (eConsent) and elect

STAT
OCTOBER 22, 2023
Roche said Monday it was buying Telavant Holdings for $7.1 billion upfront, picking up a promising experimental therapy for inflammatory bowel diseases. Telavant is owned by Roivant Sciences, with Pfizer holding a minority stake. The deal will allow Roche to develop the therapy, called RVT-3101, and, pending approval, sell it in the United States and Japan.

PharmaTech
OCTOBER 22, 2023
Webinar Date/Time: Thursday, Nov 30, 2023 at 11:00 AM EST
Let's personalize your content