SAEM Clinical Images Series: Back Yard Football Injury
ALiEM - Pharm Pearls
AUGUST 7, 2023
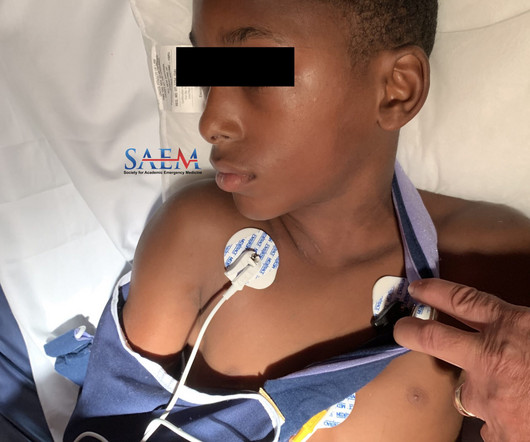
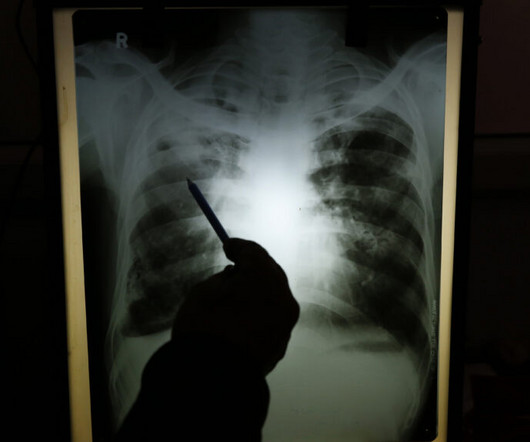
A 10-year-old male with no past medical history presents to the Emergency Department (ED) by EMS for evaluation of an injury sustained while playing tackle football. The patient was forcibly hit by another child against a tree. He complains of sharp right shoulder and chest pain that worsens with movement of his right upper extremity and he arrives wearing a sling to immobilize the arm.



































Let's personalize your content