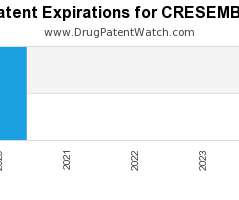
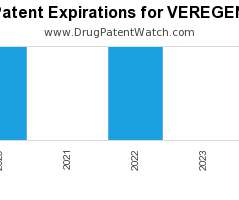
Pharma 2030: Will payers fund more preventive care programmes?
pharmaphorum
OCTOBER 26, 2020
Preventive care programmes are key to better health outcomes, but several factors are limiting their uptake. A new analysis looks at these barriers and asks how they could be overcome. Over the past decade, many healthcare systems have worked to expand their focus on health maintenance and disease prevention as several studies have indicated that preventive care services can play a significant role in improving general health while helping reduce long term healthcare costs.





























Let's personalize your content