How digital health is changing the relationship between patients and health providers
PharmaVoice
JULY 18, 2022
The pandemic has forced the pharmaceutical industry to look at digital in a new way - and it’s driving better health outcomes

PharmaVoice
JULY 18, 2022
The pandemic has forced the pharmaceutical industry to look at digital in a new way - and it’s driving better health outcomes

PharmExec
JULY 20, 2022
Thursday, July 21st 2022 at 11am EST, 8am PST, 5pm CEST Join this webinar to hear industry thought leaders discuss how data and experiences serve clinical and commercial needs, explore which platforms and technologies can enable a robust digital health ecosystem, and share recommendations for building a digital health strategy fit for your business.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.
pharmaphorum
JULY 21, 2022
Health coaching ecosystem YourCoach Health and digital therapeutics company Twill (formerly Happify Health ) are teaming up to offer access to YourCoach’s cross-specialty health coaches via Twill’s Duet platform, the companies announced today. “Digital therapy has been around for a really long time,” YourCoach Cofounder and CEO Marina Borukhovich told pharmaphorum.

Pharmaceutical Technology
JULY 19, 2022
The US Food and Drug Administration (FDA) has granted approval for Incyte’s Opzelura (ruxolitinib) cream 1.5% as a topical treatment of nonsegmental vitiligo in adults and paediatric patients aged 12 years and above. Opzelura is a topical formulation of a Janus kinase (JAK) inhibitor. With the latest development, Opzelura has became the first treatment for repigmentation in patients with vitiligo to receive FDA approval.

Speaker: Chris Antypas and Josh Halladay
Access to limited distribution drugs and payer contracts are key to pharmacy expansion. But how do you prepare your operations to take the next step? Meaningful data: Collect and share clinical data regarding outcomes, utilization, and more Reporting: Limited distribution models require efficient tracking and reporting systems Workflows: Align workflows with specific pharma and payer contractual requirements For in-depth, expert insights on pharmacy expansion, watch this webinar from Inovalon.

PharmaVoice
JULY 19, 2022
With R&D costs on the rise, biotechs are embracing new strategies and technologies to make spending more efficient.

Pharma Times
JULY 22, 2022
The treatment has been developed for people aged six years and over will be available for use across the NHS
Pharmacist Digest brings together the best content for pharmacists from the widest variety of industry thought leaders.

Pharmaceutical Technology
JULY 20, 2022
A key factor of Pfizer’s Covid-19 antiviral Paxlovid efficacy has been early intervention, but getting it in time has proven to be a challenge. On 6 July, in an effort to accelerate access, the US Food and Drug Administration (FDA) allowed pharmacists to also begin prescribing the drug to eligible individuals with Covid-19. Previously, only licensed and authorised physicians, advanced practice registered nurses, and physician assistants could prescribe the drug. .

PharmaVoice
JULY 20, 2022
Mei Mei Hu is looking to lead a third biologic ‘revolution’ by developing vaccines for chronic diseases.

PharmExec
JULY 22, 2022
Data from over 130 million quarterly HCP-field interactions across 80% of global biotech and pharma companies unveiled in Veeva Pulse Field Trends Report - industrywide data shows frequent use of key digital channels is vital for effective HCP engagement.
pharmaphorum
JULY 20, 2022
It has suspected for many years that some diseases may be linked to non-coding or ‘junk’ DNA, but the mechanism behind the pathology hasn’t been worked out. Now, scientists in the UK think they have found a culprit implicated in cancer. Junk DNA is a term used to describe the 97% of the genetic sequence in human cells found between the 3% coding for our 20,000 genes, once thought to be inert.

Advertisement
Are you still using workarounds to manage your daily operations? To achieve peak performance, it's time to explore other options for specialty and infusion pharmacy software. Streamline pharmacy operations and improve clinical performance with automated processing, real-time data exchange, and electronic decision support. Download this helpful infographic to: Drive efficiency and patient adherence from referral receipt to delivery and ongoing care – all with our Pharmacy Cloud.

Pharmaceutical Technology
JULY 20, 2022
Researchers at Gladstone Institutes and UC San Francisco (UCSF) have discovered in a study that bromodomain and extraterminal (BET) proteins are vital for the body to fight Covid-19 infection. . The research also found that BET proteins play two distinct roles in affecting how the SARS-CoV-2 virus interacts with human cells. . They provide the virus with a pathway into cells while aiding cells to defend themselves.

PharmaVoice
JULY 21, 2022
Both J&J and Novartis are making — or considering — big shifts in their fundamental company makeup.
Pharma Times
JULY 19, 2022
Imvanex is a non-replicating smallpox vaccine developed in collaboration with the US government

pharmaphorum
JULY 22, 2022
Drugmakers Pfizer and Flynn Pharma have been fined £70 million ($84 million) by the UK Competition and Markets Authority (CMA) for overcharging the NHS for a widely-used epilepsy drug. The CMA delivered a preliminary judgment in the case last year which concluded that Pfizer and Flynn abused a dominant position in phenytoin sodium capsules, causing NHS spending on the drug to balloon from around £2 million a year in 2012 to £50 million the following year.

Pharmaceutical Technology
JULY 21, 2022
AstraZeneca has signed a deal with the Federal Office of Public Health (FOPH) of Switzerland to deliver over 1,200 doses of antibody therapy, tixagevimab and cilgavimab combination (AZD7442), for Covid-19 prevention and treatment. Tixagevimab and cilgavimab are two long-acting antibodies (LAABs) obtained from the B-cells of patients convalescing following Covid-19.
PharmaVoice
JULY 20, 2022
A look at how the COVID-19 pandemic exacerbated one of the world’s greatest public health concerns.
European Pharmaceutical Review
JULY 20, 2022
Researchers have shown that ratio of nicotinic acid (NA) to nicotinamide (NAM) in cell cultures significantly increases with the presence of live microorganisms within 24 hours, acting as a useful biomarker for the detection of early-stage microbial contamination in cell therapies. Cell therapies are emerging as promising therapeutic modalities across a range of indications, with mesenchymal stromal cells (MSCs) the most clinically studied cell therapy platform worldwide; however, the nutrient r

pharmaphorum
JULY 18, 2022
GSK’s consumer health spinout Haleon started trading on the London Stock Exchange this morning, making its debut with a price of 330 pence and a market valuation of around £31 billion ($37 billion). The new company – which has achieved the largest London listing in a decade – has annual sales of around £10 billion from brands like Sensodyne toothpaste, Voltaren and Panadol painkillers, and Centrum multivitamins, making it the second-largest consumer health company in the world.

Pharmaceutical Technology
JULY 22, 2022
Pipeline therapies within the diabetic macular oedema (DME) space have recently gathered interest following the American Society of Retina Specialists (ASRS) Annual Meeting, which took place on 13–16 July. The spotlight was placed on many up-and-coming pharmacotherapies for retinal diseases, one of which was AbbVie’s/Regenxbio’s gene therapy RGX-314.

PharmaVoice
JULY 21, 2022
How the mRNA-based biotech is developing the first-ever prophylactic for a devastating virus.
Pharma Times
JULY 22, 2022
Molecular clamp technology is a protein tag that stabilises a wide range of complex viral proteins

pharmaphorum
JULY 22, 2022
Amazon has accelerated its expansion into the healthcare sector with an all-cash deal to acquire One Medical, a US group that provides virtual and in-person primary care services using a subscription fee model. Amazon is buying One Medical for $18 per share, valuing the company at around $3.9 billion and making it one of the online retail giant’s largest-ever acquisitions – and by far its biggest within the health category.
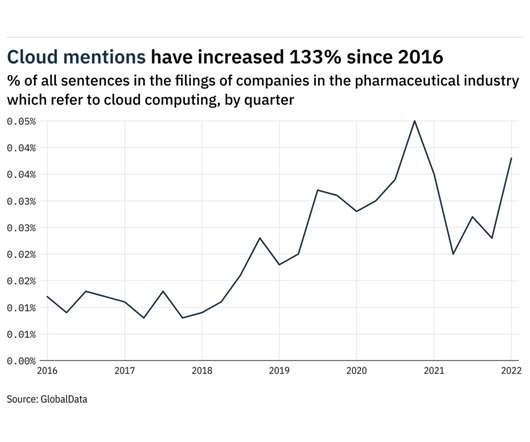
Pharmaceutical Technology
JULY 19, 2022
Mentions of cloud computing within the filings of companies in the pharmaceutical industry rose 65% between the final quarter of 2021 and the first quarter of 2022. In total, the frequency of sentences related to cloud computing between April 2021 and March 2022 was 133% higher than in 2016 when GlobalData, from whom our data for this article is taken, first began to track the key issues referred to in company filings.

PharmaVoice
JULY 19, 2022
John LaMattina has something to say about the industry — and he hopes patients are listening.

Pharma Times
JULY 20, 2022
The decentralised clinical trial centre will combine Biocorp pen injector with Aardex’s software

pharmaphorum
JULY 19, 2022
Face-to-face discussion educates regulators on what matters most to patients, and guides future decision-making. Regulators are increasingly asking drug developers to include the patient voice in submissions, but the best way to go about this is less clear-cut. In the United States, learning how to navigate forums such as the FDA’s listening sessions and patient-focused drug development (PFDD) meetings could be the key to “truly moving the dial”.

Pharmaceutical Technology
JULY 21, 2022
The pharma industry has deeply rooted environmental, social, and governance (ESG) issues that challenge sustainability. Overcoming these challenges requires collaborative, proactive steps to achieve sustainability goals and turn the reputation of this industry around. Listed below are the key technology trends impacting the ESG performance in the healthcare sector, as identified by GlobalData.

PharmaVoice
JULY 18, 2022
Learn about Digital Opinion Leaders who curate tailored content experiences and provide value to their followers.

Pharma Times
JULY 19, 2022
Specifically engineered antibody treats HER2-positive metastatic breast cancer

pharmaphorum
JULY 21, 2022
While immunotherapies have transformed treatment of many types of cancer, they do sometimes fail to have an impact – as Merck & Co has just observed in a study of Keytruda in head and neck cancer. The PD-1 inhibitor missed the mark in the phase 3 KEYNOTE-412 trial in 780 newly-diagnosed patients with head and neck squamous cell carcinoma (HNSCC), a notoriously hard-to-treat form of cancer.

European Pharmaceutical Review
JULY 18, 2022
Grünenthal has agreed to acquire testosterone treatment Nebido TM and its associated brands from Bayer AG, for up to €500 million. The long-acting injectable treatment for testosterone deficiency has been commercially approved in more than 80 countries. Nebido is also patented until March 2024 in the European Union and until May 2027 in the United States.

PharmaVoice
JULY 18, 2022
The latest executive personnel changes from around the industry.

Drug Channels
JULY 20, 2022
Cut through the steamy summer haze with our refreshing selection of articles and insights. In this issue: CVS disassociates itself from its chain pharmacy association Payers are paying attention to ICER Benefits of the biosimilar boom A fantastic takedown of health insurance deductibles Plus, I join the Advisory Board of Alto Pharmacy. P.S. Join my nearly 30,000 (!

pharmaphorum
JULY 19, 2022
Incyte’s Opzelura cream has become the first medical treatment approved to re-pigment the skin of people with vitiligo in the US, adding to its current use in atopic dermatitis. The FDA’s decision to clear the new indication for the topical JAK1/JAK2 inhibitor came after a priority review that was delayed by a request for additional data by the regulator, holding back a decision by three months.

European Pharmaceutical Review
JULY 20, 2022
Withdrawing drugs over safety concerns requires careful analysis of the health benefits and risks by regulators. 1 But while data on adverse reactions is rightly used to help with these decisions, there is currently no additional modelling on the positive impact regulatory action could have on public health. Our research paper, which is being submitted for publication this summer, aims to create an evidence-based medicine (EBM) methodology that can assess this positive impact, to further help re
Let's personalize your content