Preventing fungal contamination in pharmaceuticals
European Pharmaceutical Review
MARCH 11, 2024
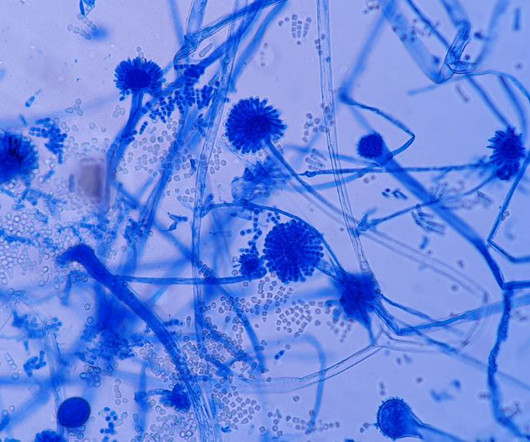
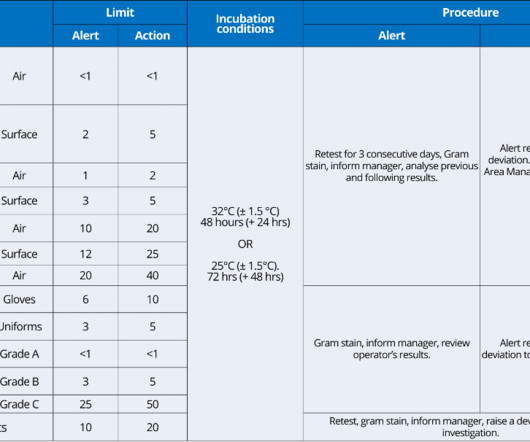
Employing MALDI-TOF MS for microbial identification in sterile drug manufacturing Fungal contamination in pharmaceutical cleaning facilities Case study examples Mould and yeast have been reported by several previous authors in numerous pharmaceutical cleanrooms , cold rooms, and controlled areas, according to the authors.















Let's personalize your content