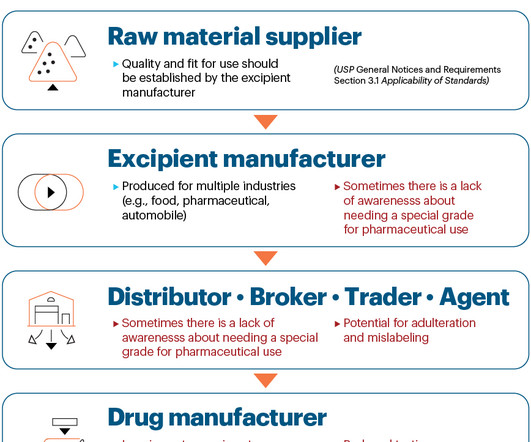
Supplier qualification standard aims to strengthen supply chains for quality medicines, dietary supplements, and foods
Quality Matters
AUGUST 3, 2023
Supplier qualification standard aims to strengthen supply chains for quality medicines, dietary supplements, and foods USP’s supplier qualification standard became official on Aug. Global supply chains are complex and vulnerable to disruption.










Let's personalize your content