Biogen tweaks confirmatory trial of Alzheimer’s drug Aduhelm
pharmaphorum
JANUARY 27, 2022
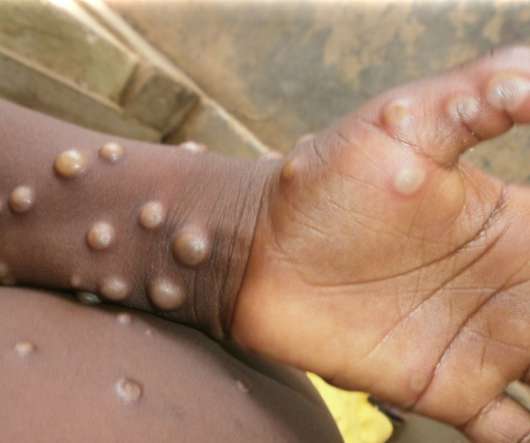
Subjects in the trial will also have to have evidence of amyloid plaques at enrolment as well as mild cognitive impairment (MCI) due to Alzheimer’s disease or mild Alzheimer’s disease, something that was required in the CMS’ initial decision but not the approved FDA labelling for Aduhelm.


















Let's personalize your content