Gilead’s Vemlidy expands label to treat paediatric chronic HBV
Pharmaceutical Technology
MARCH 29, 2024
Its label was expanded in 2022 for use in patients 12 years and older. Vemlidy was first approved to treat adults with HBV in 2016.
This site uses cookies to improve your experience. By viewing our content, you are accepting the use of cookies. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country we will assume you are from the United States. View our privacy policy and terms of use.

Pharmaceutical Technology
MARCH 29, 2024
Its label was expanded in 2022 for use in patients 12 years and older. Vemlidy was first approved to treat adults with HBV in 2016.

STAT
JANUARY 9, 2023
Although artificial intelligence is entering health care with great promise, clinical AI tools are prone to bias and real-world underperformance from inception to deployment, including the stages of dataset acquisition, labeling or annotating, algorithm training, and validation.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

STAT
JANUARY 8, 2024
The two men worked together from 2013 until 2022, when Jonas left to start a biotech incubator with funding from investment giant CBC Group. The VC firm has hired Jeff Jonas, Sage’s former chief executive, and Al Robichaud, Sage’s former chief scientific officer, as partners, the team told STAT exclusively.

European Pharmaceutical Review
OCTOBER 24, 2023
Europe’s pharmaceutical packaging and labelling market is projected to reach $35.78 billion in 2022, the pharmaceutical packaging and labelling market in Europe is expected to expand at a compound annual growth rate (CAGR) of 4.78 percent in 2022. percent in 2022. billion in 2028, according to a report by Arizton.

Pharma Pathway
SEPTEMBER 15, 2022
Intas Pharmaceuticals Ltd-Walk-In Drive for Manufacturing & Packaging Department On 18th & 25th Sept’ 2022. Experience: 03 to 09 years in Visual Inspection, Track & Trace, Labelling, E BMR, Packing Planning & Manpower Deployment. Date: 18th Sept’ 2022. Date: 25th Sept’ 2022. Job Description.

PharmaShots
FEBRUARY 1, 2023
Shots: The EC has approved a label expansion of Hemlibra (bispecific factor IXa- and factor X-directed Ab) in patients with a mod. 88.9%, respectively The decision was also based on real-world data while the label expansion will provide an effective & convenient prophylactic treatment option for patients with a mod. haemophilia A.

Digital Pharmacist
OCTOBER 24, 2022
Date of Approval: 9/30/2022. Date of Approval: 9/29/2022. Date of Approval: 9/22/2022. Date of Approval: 9/21/2022. Date of Approval: 9/14/2022. Date of Approval: 9/9/2022. Below we will be listing some of the new drug therapy advances and approvals that the FDA had in the month of September.

ALiEM - Pharm Pearls
APRIL 14, 2023
Highlighted Quality Posts: Procedures Site Article Author Date Label Rebel EM Intra Articular Lidocaine vs Sedation in Shoulder Reductions Nordia Matthews, MD 30 Jan 2023 AIR EM Docs Video Laryngoscopy in the ED Cameron Jones, MD 8 Aug 2022 AIR First 10 EM Lacerations: Does closure technique matter?

FDA Law Blog: Biosimilars
APRIL 21, 2024
But that’s the controversy here: Did FDA approve LYTGOBI NDA 214801 on September 30, 2022 when the Agency issued its initial approval letter , or on October 5, 2022 when FDA issued a corrected approval letter ? Rather, “permission for commercial marketing” was only effective upon FDA’s issuance of its corrected October 5, 2022 letter.

STAT
JANUARY 10, 2024
Food and Drug Administration responded to decades of escalating concerns about Singulair, a widely prescribed drug for asthma and allergies, by adding a stark warning on the labeling that it could cause aggression, agitation and even suicidal thought. in 2022, The New York Times writes. The

ALiEM - Pharm Pearls
OCTOBER 14, 2023
Highlighted Quality Posts: Respiratory Site Article Author Date Label EMCrit IBCC: Asthma Josh Farkas, MD April 12, 2023 AIR EMCrit IBCC: Severe Community Acquired Pneumonia Josh Farkas, MD October 11, 2022 AIR EM Docs Empyema: ED Presentation, Evaluation, and Management Heath Garner, MD April 11, 2022 AIR Rebel EM Pigtail Catheter vs Large Bore Chest (..)

FDA Law Blog: Biosimilars
APRIL 25, 2024
Promotional labeling is generally any labeling other than FDA-required labeling that is devised for the promotion of a product, as well as other functions, and can include printed, audio, or visual matter that describes the product. Comments to the revised draft guidance are due to the docket by June 25, 2024.

ALiEM - Pharm Pearls
JULY 20, 2023
Highlighted Quality Posts: Infectious Disease Site Article Author Date Label SGEM Lumbar punctures in febrile infants with positive urinalysis – it’s just overkill Dennis Ren, MD December 31, 2022 AIR EMDocs Bacterial Meningitis Mounir Contreras Cejin, MD January 28, 2023 HM ALiEM The Febrile Infant Corey Ziemba, MD, Justin Hacnik, MD and J.D.

PharmaShots
APRIL 20, 2023
treatment interruption/discontinuation due to low lgG & lgM in (0.2% & 10.3%)/ (0.2% & 3.6%) patients Ref: Novartis | Image: Novartis Related News:- Novartis Presents Results of Kesimpta (ofatumumab) in P-III (ASCLEPIOS I/II) and (ALITHIOS) Open-Label Extension Trial for RMS at EAN 2022 vs switch group (-0.42%/yr.)
pharmaphorum
JANUARY 12, 2021
Meanwhile, EMPEROR-Reduced also showed a slowdown in the rate of decline in kidney function among patients with HFrEF, an effect that Lilly and Boehringer are exploring in the CKD patient population in the EMPA-KIDNEY trial due to generate results in 2022.
pharmaphorum
JUNE 28, 2021
The duo are carrying out the EMPA-KIDNEY trial in CKD patients with and without diabetes, with results due in 2022, although it is possible that the study could be stopped early – like DAPA-CKD – if interim results are similarly strong. The post AZ closes in Forxiga kidney disease label in EU after nod from CHMP appeared first on.

Express Pharma
NOVEMBER 8, 2023
per cent across the seven major markets (7MM*) from $3 billion in 2022 to $6.7 Barbora Salcman, Neurology Analyst at GlobalData, comments, “Until 2017, all MG patients were dependant on a range of off-label treatments, such as steroids, immunosuppressants or acetylcholinesterase inhibitors, independent of their diagnosis or MG class.

Express Pharma
JUNE 26, 2023
Vishruth Srivastava, Managing Director of Yodaplus, a leading player in AI technology, asserts that AI can assist in generating label claims with up-to-date perspectives on each compound and making informed decisions regarding the necessity for further clinical trials or general precautions.
pharmaphorum
APRIL 23, 2021
Calliditas Therapeutics is on course to launch its first product, Nefecon for rare disease primary IgA nephropathy (IgAN), in the first half of 2022 after getting the green light for an accelerated review by the EMA. . The post Calliditas eyes 2022 approval in EU for rare kidney disease drug Nefecon appeared first on.

LifeProNow
MARCH 30, 2023
Drug overdose persists as a major public health issue in the United States, with more than 101,750 reported fatal overdoses occurring in the 12-month period ending in October 2022, primarily driven by synthetic opioids like illicit fentanyl. The FDA remains committed to addressing the evolving complexities of the overdose crisis.

Pharmaceutical Business Review
MARCH 28, 2024
Additionally, three open-label studies showed the drug’s efficacy and safety when administered at doses ranging between 2-6mg/kg/day for up to 30 months. The submission also encompasses pharmacokinetic data from a study involving healthy adult Chinese volunteers.

LifeProNow
DECEMBER 22, 2023
The most recent inspection of the company’s facilities in 2022 found the majority of the inspectional observations repeated those found in past FDA inspections and detailed in a 2019 warning letter. Despite repeated warnings, the company and Badia continued to violate the law.

Safe Biologics
MARCH 30, 2023
The recently-published Executive Summary from the 75th INN Consultation, at which ASBM presented in October 2022, summarizes ASBM’s position regarding the value of distinct biologic nomenclature: One significant challenge to efficient global cooperation is the lack of harmonization.

Pharma Leaders
APRIL 7, 2023
In a letter to the company, the agency did not detail the deficiencies, but said they would preclude the agency from holding discussions with the company about labeling and postmarketing commitments, Ascendis said. The FDA granted the NDA a priority review in October 2022 and the agency’s decision date is still slated for April 30.

ALiEM - Pharm Pearls
NOVEMBER 3, 2023
You will need to create a free, 1-time login account.

LifeProNow
JANUARY 19, 2024
The FDA has revised the final guidance, Submission and Review of Sterility Information in Premarket Notification (510(k)) Submissions for Devices Labeled as Sterile, to list VHP as an example of an Established Category A method of sterilization.
Pharmaceutical Technology
NOVEMBER 25, 2022
In recent months, Wegovy (semaglutide), indicated for obesity, has been subjected to widespread supply shortages due to high demand, in addition to manufacturer production problems, with Novo Nordisk intending to relaunch Wegovy by the end of 2022.

pharmaphorum
OCTOBER 14, 2022
” The programme has been set up to develop four devices – an e-paper label, smart wearable sensor, smart pill box, and endo-cutter used in surgical procedures – with funding from the EU’s Horizon Europe programme and support from the Waste Electrical and Electronic Equipment (WEEE) Forum.

Pharmaceutical Technology
DECEMBER 11, 2022
At the 2022 annual American Society of Hematology 2022 (ASH 2022) conference, being held between December 10-13, new data from the multi-arm , uncontrolled Phase I/II BRUIN study of pirtobrutinib were presented by Dr. These results pave the way for a new option for relapsed/refractory (R/R) RT patients.

Pharmaceutical Business Review
MARCH 29, 2024
The recent approval builds on a 2022 decision which allowed its use in paediatric patients aged 12 years and above. The trial results demonstrated that subjects in both the Vemlidy group and the placebo group who switched to open-label Vemlidy after 24 weeks, experienced significant improvements.
European Pharmaceutical Review
JUNE 28, 2022
The Phase III Asclepios I/II trials and the Alithios open-label extension from Novartis , showed that earlier initiation with Kesimpta resulted in a more than three-fold increased likelihood of maintaining NEDA-3 throughout the study. The data were presented at the 2022 European Academy of Neurology (EAN) Annual Meeting.
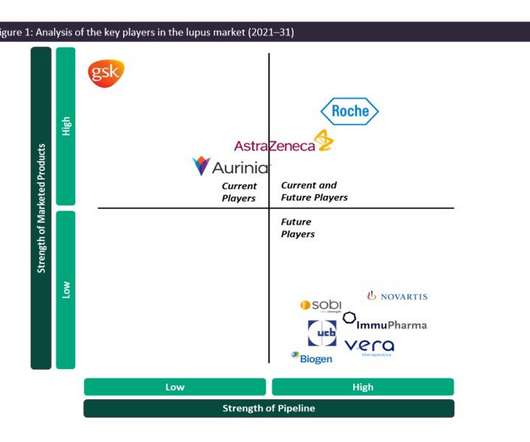
Pharmaceutical Technology
JANUARY 23, 2023
The results of this trial, if positive, are expected to be sufficient to grant approval in the US and EU through a supplemental new drug application and label expansion, respectively. in 2018 across the 7MM despite being an off-label therapy for lupus.

Pharmaceutical Business Review
JANUARY 10, 2024
Produodopa received a marketing authorisation through the Decentralized Procedure in the third quarter of 2022, following the positive outcomes of three supporting studies. The M15-741 study, a Phase III 12-month open-label trial, assessed the long-term tolerability, safety, and efficacy of Produodopa.

RX Note
MARCH 18, 2023
NOTE: The latest MOHMF database is 2022-3. Initiatives under Rx Note Rx via AppSheet or Caspio Rx & Calc via Open As App MOH Medicines Formulary (FUKKM) via Wistify Database last updated to MOHMF 2022-3. Bluebook/NAG KKM Malaysia under DevTechMonster Database last updated to MOHMF 2022-2.

European Pharmaceutical Review
SEPTEMBER 5, 2023
It was in February 2022 that NICE recommended the obesity treatment in its draft guidance. Novo Nordisk’s obesity treatment is also expected to be filed for a label expansion in the US this year. Prior to this new market launch, Wegovy was only available in Norway, Denmark, Germany and the US.

Pharmaceutical Technology
JUNE 7, 2023
In 2022, Lilly reported sales for Mounjaro were approximately $500m, but this is expected to increase significantly in 2023 due to strong demand for the T2D drug. Mounjaro’s approval in 2022 for T2D has already led to many prescribers providing the therapy off-label to their patients to help them lose weight.

RX Note
JUNE 8, 2023
This book received highly recommended recognition in Obstetrics and Gynaecology at the British Medical Association (BMA) Medical Book Awards in 2018 and selected as a Doody's Core Title for 2022 and 2023. In the past, package inserts were not always reliable sources of information regarding medication safety during lactation.
pharmaphorum
AUGUST 16, 2022
Lynparza was approved in 2020 as a second-line therapy for homologous recombination repair (HRR) gene-mutated mCRPC last year, but extending its label to include non-HRR patients in the frontline setting would dramatically increase the number of patients eligible for treatment. months and 16.6 months, respectively.

pharmaphorum
OCTOBER 14, 2022
Now, the label says it “should be removed or replaced after eight years at the latest,” according to Bayer. The label extensions come on the back of a clinical trial involving around 3,330 women that showed that Mirena’s contraceptive efficacy remains high, at more than 99% during years six to eight years of use.
Pharmaceutical Technology
MAY 16, 2023
In July 2022, the US FDA accepted the company’s BLA for its investigational next-generation anti-HER2 antibody-drug conjugate, SYD985, to treat HER2-positive unresectable locally advanced or metastatic breast cancer (MBC) patients.

Pharmaceutical Technology
APRIL 27, 2023
The treatment will go up against Sanofi’s Nexviazyme (avalglucosidase alfa-ngpt) which received an EC approval in June 2022, following a US Food and Drug Administration ( FDA) approval in August 2021. Sanofi markets avalglucosidase alfa as Lumizyme in the US to treat LOPD since its approval in 2010.

Big Molecule Watch
APRIL 11, 2024
CARVYKTI was originally approved by FDA in February 2022 for treatment of adult patients with relapsed or refractory multiple myeloma after four or more prior lines of therapy. The post FDA APPROVES BMS AND J&J CAR-T CELL THERAPIES FOR THE EARLIER TREATMENT OF MULTIPLE MYELOMA appeared first on Big Molecule Watch.

Pharma Marketing Network
MARCH 25, 2022
Pharma can help with caregiver-relevant labeling, training to perform nursing duties, and a multitude of other support services. Accessed March 21, 2022. Accessed March 12, 2022. Accessed March 15, 2022. Accessed February 28, 2022. Accessed March 12, 2022. Accessed March 17, 2022. February 2019.
European Pharmaceutical Review
MARCH 8, 2023
An open-label, Phase IIa, proof-of-concept clinical trial in women with PPD is expected to initiate in the second half of 2023. A placebo-controlled, Phase IIa, proof-of-concept, social anxiety clinical trial is expected to begin in the first half of 2023. The results are anticipated by the end of 2023.
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?


Let's personalize your content