Boehringer, Lilly’s Jardiance ties to match AZ’s Farxiga with heart failure label
pharmaphorum
JANUARY 12, 2021
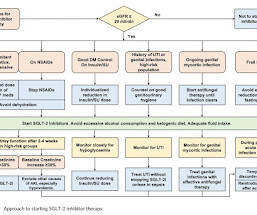
Boehringer and Eli Lilly have moved closer to a heart failure indication for their SGLT2 inhibitor Jardiance, as the FDA starts a fast-track review of the drug in its first use beyond diabetes. Invokana was the first mover among the SGLT2 drugs in the kidney area, winning FDA approval towards the end of 2019 for diabetic kidney disease.


































Let's personalize your content