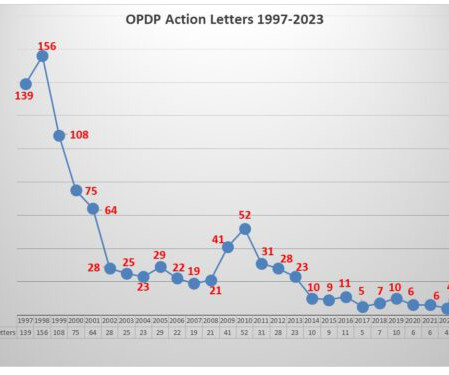
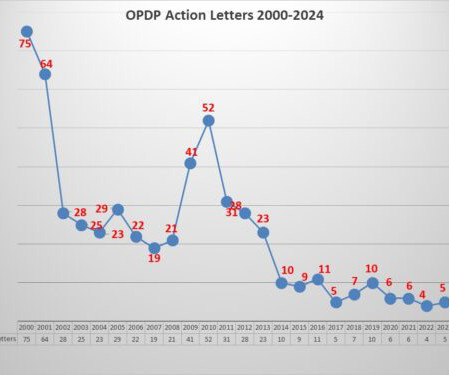
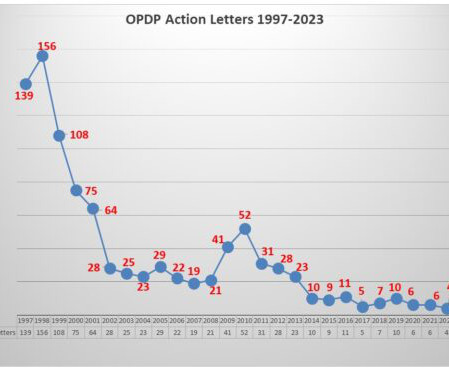
FDA’s OPDP Issues Third Letter of 2023
Eye on FDA
AUGUST 22, 2023
The Untitled Letter posted this week involved an oral birth control pill which has a specific of contraindications contained in the label as well as a list of warnings and precautions and of the most common adverse events. The communications vehicle in question was a social media sponsored posting. Pharma communicators take note.



































Let's personalize your content