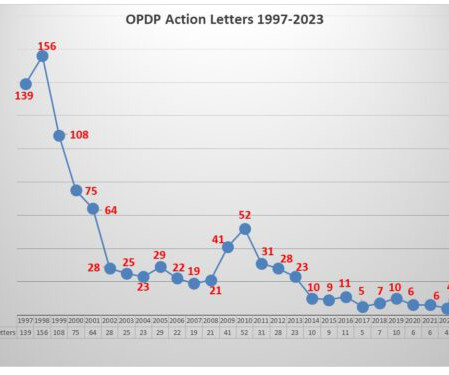
FDA’s OPDP Issues Third Letter of 2023
Eye on FDA
AUGUST 22, 2023
The communications vehicle in question was a social media sponsored posting. OPDP took issue with three specific aspects of the promotional communication. First, while presenting the indication in the posting, there was no risk information included in the communication. Pharma communicators take note.



































Let's personalize your content