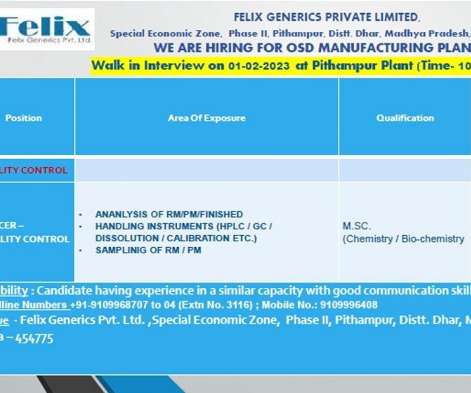
Felix Generics Pvt. Ltd.-Walk-In Interview for Quality Control On 1st Feb’ 2023
Pharma Pathway
JANUARY 29, 2023
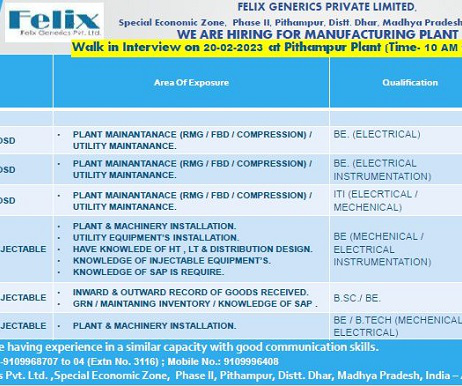
Felix Generics Pvt. Walk-In Interview for Quality Control On 1st Feb’ 2023 Job Description Felix Generics Pvt. is a pharmaceutical generics business Company. HPRA Approved (April 2021) facility for veterinary generic medicines. Walk-In Interview for Quality Control On 1st Feb’ 2023



























Let's personalize your content