Biosimilars are gaining ground. The IRA could push them even further next year.
PharmaVoice
MAY 13, 2024
As commercial momentum builds, coverage incentives for the Medicare market are expected to favor biosimilars in 2025.
This site uses cookies to improve your experience. By viewing our content, you are accepting the use of cookies. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country we will assume you are from the United States. View our privacy policy and terms of use.

PharmaVoice
MAY 13, 2024
As commercial momentum builds, coverage incentives for the Medicare market are expected to favor biosimilars in 2025.
STAT
SEPTEMBER 23, 2022
Market share is often held up as the most relevant metric for the success of a biosimilar class. I believe there are other metrics, like cost savings or signs of greater patient access, that should also be used to define biosimilars’ successes or failures. the first biosimilar was launched in September 2015. In the U.S.,
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

pharmaphorum
AUGUST 24, 2022
Amgen has reported positive phase 3 results with its biosimilar version of AstraZeneca/Alexion’s blockbuster rare disease drug Soliris, setting up a regulatory filing with the FDA. The safety and immunogenicity profile of the biosimilar was also comparable to Alexion’s drug, said Amgen.

BioPharma Dive
MAY 13, 2024
As commercial momentum builds, coverage incentives for the Medicare market are expected to favor biosimilars in 2025.

Big Molecule Watch
MARCH 27, 2024
The Biden Administration recently released a 2025 Budget Proposal which includes permitting biosimilar substitution without the Food and Drug Administration’s (“FDA”) separate determination of interchangeability. Specifically, the U.S. According to HHS, “[t]his change makes the U.S. According to HHS, “[t]his change makes the U.S.

Big Molecule Watch
APRIL 22, 2024
On April 16, Alvotech and Teva announced the FDA approval of SELARSDI (ustekinumab-aekn), biosimilar to Johnson and Johnson’s STELERA® (ustekinumab). SELARSDI is now the second ustekinumab biosimilar to be approved by the FDA, following FDA approval of Amgen’s WEZLANA (ustekinumab-auub) in October 2023.

European Pharmaceutical Review
NOVEMBER 2, 2023
The US Food and Drug Administration ( FDA) has approved Wezlana (ustekinumab-auub) as a biosimilar to Johnson & Johnson’s Stelara (ustekinumab). Sustainable biosimilar competition in Europe: can it be achieved? Stelara biosimilars in the US market The first Stelara biosimilars are expected to enter the US market in 2025. “We

Big Molecule Watch
MARCH 6, 2024
regarding YESAFILI, Biocon’s proposed biosimilar to EYLEA (aflibercept). Under the agreement, Biocon can launch YESAFILI in Canada no later than July 1, 2025. patent litigation related to biosimilars of EYLEA (aflibercept) can be found on the Big Molecule Watch’s BPCIA Litigation Tracker. On March 4, Biocon Biologics Ltd.

Big Molecule Watch
MARCH 6, 2024
and Johnson & Johnson (“J&J”) regarding Bmab 1200, Biocon’s proposed biosimilar to STELARA. The agreement allows Biocon to launch in the United States in February 2025, pending FDA approval. Market Entry Date for Ustekinumab Biosimilar appeared first on Big Molecule Watch. Amgen secured a U.S.

Big Molecule Watch
FEBRUARY 1, 2024
Celltrion Submits BLA for Tocilizumab Biosimilar On January 28, 2024, Celltrion USA announced that it has completed submission of its application to the FDA for CT-P47, its proposed tocilizumab biosimilar. FDA Accepts Accord BioPharma’s BLA for Ustekinumab Biosimilar On January 4, 2024, Accord BioPharma, Inc.
European Pharmaceutical Review
JUNE 9, 2023
Samsung Biologics, the South Korean contract development and manufacturing organisation (CDMO), has entered into a strategic partnership for the long-term commercial manufacturing of Pfizer’s multi-product biosimilars portfolio. The contact is valued at some $411 million, according to a company filing.

Express Pharma
FEBRUARY 29, 2024
Biocon Biologics has signed a settlement and license agreement with Janssen Biotech, and Johnson & Johnson (collectively known as Janssen) that clears the way to commercialise its Bmab 1200, a proposed biosimilar to Stelara, in the US. The agreement licenses the company to launch in the US, in February 2025, once approved by the US FDA.

pharmaphorum
FEBRUARY 23, 2022
Adoption of biosimilars in the US has been notoriously slow, but biosimilars manufacturers and outside observers expect this to change rapidly in the coming years. One way in which the pharmaceutical industry and regulators have reacted is by developing or facilitating the development of biosimilars. The short story so far.

Big Molecule Watch
SEPTEMBER 28, 2023
Insulin Biosimilars: Meitheal Pharmaceuticals, Inc. to commercialize Tonghua Dongbao’s three insulin biosimilars (insulin aspart, insulin lispro, and insulin glargine) in the U.S. Oncology, Women’s Health, and Respiratory Disease Biosimilars: Abbott has announced an expansion of its agreement with mAbxience Holdings S.L.
pharmaphorum
FEBRUARY 28, 2022
India’s Biocon has expanded its pipeline of biosimilars with a $3.3 Biocon’s partnership with Mylan – which merged with Pfizer’s Upjohn to form Viatris in 2020 – dates back more than a decade and has generated a number of biosimilars including insulin analogues, antibodies and recombinant proteins.

STAT
AUGUST 30, 2023
government’s initial approach to negotiating drug prices could discourage cheaper biosimilar versions of more complex pharmaceuticals that eat up a high portion of total Medicare spending , Bloomberg Law explains. Amgen and J&J reached an agreement in May to allow a biosimilar for Stelara no later than Jan.
Big Molecule Watch
FEBRUARY 23, 2024
On February 15, Alvotech announced that it reached more settlement agreements with Johnson & Johnson for ATV04, Alvotech’s biosimilar to STELARA (ustekinumab). In Canada, their partner is JAMP Pharma Group, and the biosimilar will be marketed as JAMTEKI. subject to regulatory approval, no later than February 21, 2025.

Express Pharma
OCTOBER 10, 2023
The patent for Janssen’s second-generation, tumour necrosis factor-alpha (TNF)-a inhibitor Simponi (golimumab), is scheduled to expire in 2024 and in 2025 in the US and EU, respectively. This global study positions AVT05 as the leading contender for Simponi biosimilars in the rheumatoid arthritis (RA) market, says GlobalData.

Big Molecule Watch
DECEMBER 13, 2023
Samsung Bioepis recently reported that it has signed a settlement and license agreement with Johnson & Johnson (“J&J”) in the United States relating to SB17, Samsung Bioepis’s ustekinumab biosimilar to J&J’s STELARA®. If SB17 is approved by the FDA, the license period in the United States will begin on February 22, 2025.
pharmaphorum
SEPTEMBER 20, 2022
Novartis’ soon-to-be-divested Sandoz unit has positive clinical trial data in hand for a biosimilar of Amgen’s blockbuster osteoporosis therapy Prolia (denosumab), clearing the way for regulatory filings. Other companies working on biosimilar versions of denosumab include Samsung Bioepis, Celltrion, and AryoGen Pharmed.

Big Molecule Watch
MAY 18, 2023
The USPTO has proposed several fee changes for implementation in 2025. The post Proposed USPTO Fee Changes Could Impact PGR and IPR Filing Strategies Used by Biosimilars appeared first on Big Molecule Watch. To submit comments via the portal, commenters should enter docket number PTO–P–2023–0017 on the homepage.

Pharmacy Times
OCTOBER 18, 2023
Jensen notes that although she expects there will be a lot of FDA approvals in 2023 and 2024, there likely will not be another biosimilar “boom” until 2025.

PharmaShots
FEBRUARY 1, 2023
Ximluci is expected to be available in the UK in 2023 The approval for Ximluci was granted through the EC decision reliance procedure, whereby the MHRA’s decision was based on the EC Ximluci was approved in the UK for wet AMD, DME, diabetic retinopathy, RVO, and visual impairment due to choroidal neovascularization in adults.
Express Pharma
SEPTEMBER 7, 2023
Adalimumab biosimilars are reshaping the landscape, impacting Humira and competitors like Janssen’s Stelara and Takeda’s Entyvio. However, as with Humira, revenues from these therapies are expected to decline with the release of their corresponding biosimilars, which are anticipated to enter the market in the coming years.

Pharmaceutical Technology
AUGUST 8, 2023
The deal sets the biosimilar’s potential US launch date to no later than April 2025, following two agreements signed earlier this year.

Big Molecule Watch
NOVEMBER 6, 2023
On Tuesday, October 31, FDA approved Amgen’s WEZLANA (ustekinumab-auub) as biosimilar to and interchangeable with Janssen’s STELARA (ustekinumab). WEZLANA is the first product to be approved as a biosimilar to STELARA.

Big Molecule Watch
AUGUST 30, 2023
It has been reported that Celltrion has finalized a settlement with Johnson & Johnson (“J&J”) in the United States relating to CT-P43, Celltrion’s ustekinumab biosimilar to J&J’s STELARA®, which would permit Celltrion to launch the product in the U.S. market on March 7, 2025, if approved by FDA.
Big Molecule Watch
JUNE 13, 2023
announced that they reached a settlement and license agreement with Johnson & Johnson regarding AVT04, Alvotech’s proposed biosimilar to STELARA (ustekinumab) in the United States. According to the settlement agreement, AVT04 (ustekinumab) can be marketed in the US, subject to regulatory approval, no later than February 21, 2025.”

European Pharmaceutical Review
FEBRUARY 27, 2024
Novel biosimilar approval In the same month, the US Food and Drug Administration (FDA) approved Amgen’s Wezlana (ustekinumab-auub) as the first biosimilar to reference blockbuster drug Stelara (ustekinumab). The post Amgen opens its most advanced manufacturing facility to date appeared first on European Pharmaceutical Review.

Big Molecule Watch
AUGUST 16, 2023
On August 7, 2023, Formycon AG and Fresenius Kabi announced that they have reached a settlement with Johnson & Johnson (“J&J”) in the United States relating to FYB202, a proposed ustekinumab biosimilar to STELARA®, marketed by J&J. Economic terms of the settlement were not disclosed in the press release.

Express Pharma
JULY 3, 2023
As the demand for biologics and biosimilar drugs grows, an even greater degree of analytical data is sought, which is one reason for our recent acquisition of light-scattering leader, Wyatt Technology. The biosimilar market in India is estimated to grow at 22 per cent CAGR to become $12 billion by 2025.

STAT
NOVEMBER 1, 2023
The FDA approved Amgen’s biosimilar version of Johnson & Johnson’s blockbuster psoriasis treatment, Stelara, for multiple inflammatory diseases , Reuters says. But Public Citizen maintains Medicare officials should consider the ongoing cost of the patenting tactics when negotiating a price. billion in 2022.

Express Pharma
JANUARY 12, 2024
With the Indian Pharma sector trying to reach the USD 130 Billion target by 2030, there is a renewed spirit of research in the areas of cell and gene therapy, biologics and biosimilars apart from the already strong generic and vaccine manufacturing sectors of the country.

Express Pharma
JANUARY 11, 2024
GlobalData’s latest report, “Gout Market Opportunity Assessment, Epidemiology, Clinical Trials, Unmet Needs and Forecast to 2032,” reveals that there are nine therapies in the late-stage pipeline, most of which are urate-lowering therapies (ULTs) entering the US and Japan markets from 2025 onwards.

Big Molecule Watch
APRIL 17, 2023
CT-P39 is a biosimilar referencing Xolair (ingredient: omalizumab), which is an antibody biopharmaceutical that treats allergic asthma, chronic urticaria, and chronic rhinosinusitis. Xolair’s substance patent has already expired and the formulation patent is set to expire in March 2024 in Europe and November 2025 in the U.S.

Express Pharma
JULY 4, 2023
The latest deals will see the biotech division of the Samsung Group produce biosimilar products ranging from oncology and inflammation to immunotherapy in the period to 2029 at its new Plant 4 in South Korea. billion) through September 2025 to build a new factory in South Korea. billion, Samsung Biologics said in a statement.

Big Molecule Watch
JANUARY 25, 2024
As we kick off 2024, we reflect on regulatory developments in the biologics and biosimilars space in 2023. The approval of Amgen’s WEZLANA (ustekinumab-auub) as biosimilar to Janssen’s STELARA (ustekinumab) is noteworthy given that it received designation as interchangeable. Below are some of the top regulatory developments from 2023.

pharmaphorum
DECEMBER 13, 2020
Boston, US-based Alexion spent a lot of 2019 arguing the merits of remaining independent, saying that while Soliris is approaching the end of its patent life – with heavyweight competitors like Amgen already eyeing the biosimilar market for the drug – Ultomiris and its pipeline could help drives sales to $9 to $10 billion in 2025.
pharmaphorum
OCTOBER 13, 2022
ResearchandMarkets also recently published a report , which highlighted only biosimilars as the emerging treatments for osteoporosis. Emerging research and biosimilars. Sandoz recently announced positive clinical trial results from a phase 1/3 into its biosimilar to denosumab.
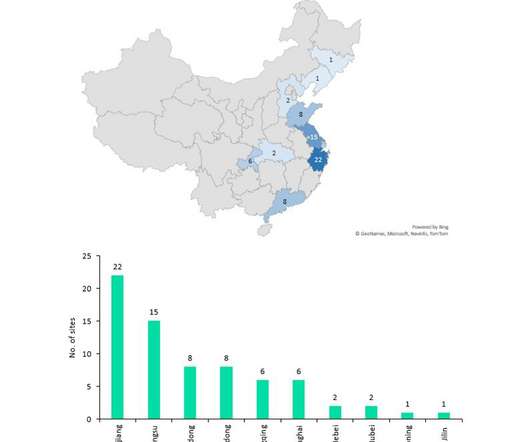
Pharmaceutical Technology
NOVEMBER 22, 2022
Despite China producing a significant proportion of the world’s API supply (mostly small molecule), it manufactures relatively few biosimilar and innovator drugs and no cell and gene therapies for the western markets of Europe and the US despite investments and an increasing number of startups to improve innovative manufacture.

Pharmaceutical Technology
MAY 19, 2023
Rinvoq is a major pillar in AbbVie’s plan to stem revenue drops once biosimilars to its behemoth Humira (adalimumab) enter the market on a sustained basis this year. AbbVie anticipates Rinvoq sales to exceed $7.5bn in 2025 and for the peak revenues from both drugs to exceed Humira’s peak sales by 2027.

Big Molecule Watch
JUNE 29, 2023
As we previously reported , Biogen sued Sandoz and Polpharma (“Defendants”) in a BPCIA litigation related to Defendants’ natalizumab biosimilar. The Court also noted that Biogen’s own expert cited sources showing that, in the 12-18 months after biosimilar launch, the price of the biosimilar product remained stable.

pharmaphorum
DECEMBER 21, 2020
Boston, US-based Alexion spent a lot of 2019 arguing the merits of remaining independent, saying that while Soliris is approaching the end of its patent life – with heavyweight competitors like Amgen already eyeing the biosimilar market for the drug – Ultomiris and its pipeline could help drives sales to $9 to $10 billion in 2025.
Express Pharma
NOVEMBER 2, 2023
Though the entry of biosimilars will contract the market, significant growth is expected over the next 8-10 years, driven by the launch of new assets. The anticipated launches in the US and Europe of Janssen’s Tremfya (guselkumab) and Lilly’s mirikizumab, in Q4 2025 and Q3 2026, respectively, are expected to shape the market.
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?


Let's personalize your content