FDA Fast Track designation for photodynamic cancer therapy
European Pharmaceutical Review
NOVEMBER 28, 2022
The drug was evaluated in four Phase II/III clinical trials in patients previously given chemotherapy and/or failed radiation therapy. FTD facilitates drug development and can expedite the review of drugs for serious conditions to help address unmet medical needs.







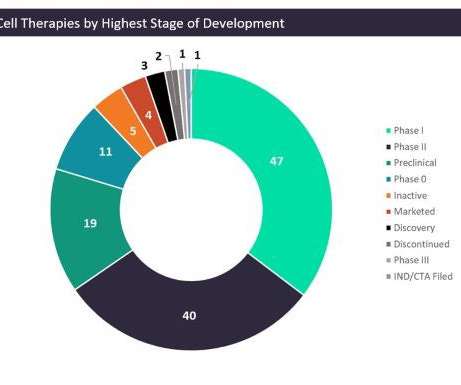







Let's personalize your content