Cannabinoids show promise in acute migraine clinical trial
pharmaphorum
MARCH 4, 2024
Inhaled cannabinoids have been shown to perform better than placebo in providing pain relief for people suffering from acute migraine in a clinical trial
This site uses cookies to improve your experience. By viewing our content, you are accepting the use of cookies. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country we will assume you are from the United States. View our privacy policy and terms of use.

 clinical migraine
clinical migraine 
pharmaphorum
MARCH 4, 2024
Inhaled cannabinoids have been shown to perform better than placebo in providing pain relief for people suffering from acute migraine in a clinical trial

European Pharmaceutical Review
SEPTEMBER 14, 2023
Rimegepant (Vydura) is the “first and only NICE-recommended medicine that can help alleviate the misery of acute migraines, and may be considered a step-change in treatment,” shared Helen Knight, Director of medicines evaluation at the National Institute for Health and Care Excellence (NICE).
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.
Pharmafile
DECEMBER 6, 2023
Teva Pharmaceuticals has announced data from a post hoc analysis of two phase 3 clinical studies around the effectiveness of migraine prevention treatment, Ajovy (fremanezumab) in the reduction of migraine attacks in patients with migraine and co-morbid obesity.

European Pharmaceutical Review
NOVEMBER 17, 2023
Results from AbbVie’s Phase III study, PRODROME , published in The Lancet showed that UBRELVY ® (ubrogepant) 100mg for acute treatment of migraine significantly reduced the likelihood of development of moderate or severe headache compared to placebo within 24 hours post-dose.

Pharmaceutical Technology
JUNE 1, 2023
The UK’s National Institute for Health and Care Excellence (NICE) has issued final draft guidance recommending the use of Pfizer ’s rimegepant (Vydura) to prevent migraine attacks. NICE medicines evaluation director Helen Knight said: “Each year, the lives of millions of people in England are blighted by migraine attacks.

LifeProNow
MARCH 10, 2023
FDA has approved ZAVZPRET™ (zavegepant), the first and only calcitonin gene-related peptide (CGRP) receptor antagonist nasal spray for the acute treatment of migraine with or without aura in adults. When a migraine hits, it has a significant negative impact on a person’s daily life,” said Kathleen Mullin, M.D.,

European Pharmaceutical Review
AUGUST 17, 2023
Recent EU approval means adults who have four or more migraine days per month can now access the first and only once-daily oral calcitonin gene-related peptide (CGRP) receptor antagonist (gepant). This was compared to 27 percent of patients in the placebo arm. This result was compared to 29 percent of participants given placebo.
Express Pharma
NOVEMBER 17, 2023
Dr Reddy’s Laboratories announced the roll-out of Nerivio in India, a state-of-the-art United States Food and Drug Administration (USFDA) approved wearable therapy device for drug-free management of migraine. It also has an interactive migraine diary which can be used to log symptoms, track responses and share insightful analytics.

European Pharmaceutical Review
JUNE 1, 2023
An oral treatment for preventing migraines has been recommended by the National Institute for Health and Care Excellence (NICE) for the first time. Rimegepant (Vydura) is recommended as an option for episodic migraine in adults after having at least three previous failed preventive treatments. How does the oral migraine treatment work?

Pharmaceutical Technology
MARCH 10, 2023
The US Food and Drug Administration (FDA) has granted approval for Pfizer ’s Zavzpret (zavegepant) for the acute treatment of migraine in adult patients with or without aura. Zavzpret is claimed to be the first and only calcitonin gene-related peptide (CGRP) receptor antagonist nasal spray approved to treat migraine.

PharmaShots
JUNE 22, 2023
The results showed no statistical difference b/w women with migraine who used the device during pregnancy throughout 3mos. The device is a safe treatment for migraine during pregnancy, not increasing the risk for adverse pregnancy outcomes. postpartum, and for those who did not use during this period, 66.7% achieved pain relief @2hrs.

Pharmafile
SEPTEMBER 5, 2023
AbbVie has announced that the Medicines and Healthcare products Regulatory Agency (MHRA) has granted a marketing authorisation for Aquipta (atogepant), for the prophylaxis of migraine in adult patients who have had at least four migraine days each month.

Pharmacy Times
JUNE 30, 2023
Galcanezumab-gnlm (Emgality; Eli Lilly and Company) did not demonstrate superiority to rimegepant orally disintegrating tablet (Nurtec ODT) on the percentage of individuals achieving a 50% or greater reduction in monthly migraine headache days.
STAT
SEPTEMBER 14, 2022
… For pharmaceutical companies, online prescribing has now become a powerful tool to drive sales, sending hundreds of thousands of patients to the clinic at the moment they are most primed to ask for a specific prescription, STAT reports.

Pharmaceutical Technology
OCTOBER 4, 2022
A CGRP receptor antagonist, NURTEC ODT is approved by the US Food and Drug Administration (FDA) for use in adults for acute treatment of migraine irrespective of aura status as well as for preventive episodic migraine treatment. It is also approved in the EU under the trade name Vydura for use in similar indications.

Pharmaceutical Technology
MAY 19, 2023
The US Food and Drug Administration (FDA) has accepted Satsuma Pharmaceuticals’ 505(b)(2) new drug application (NDA) for STS101 for acute treatment of migraine, for review. It provides significant benefits compared to the current acute treatments for migraine.

Pharmaceutical Technology
NOVEMBER 18, 2023
LuAG-09222 is under clinical development by H. Lundbeck and currently in Phase II for Migraine.

Pharmaceutical Technology
FEBRUARY 8, 2024
Prabotulinumtoxin A biosimilar is under clinical development by AEON Biopharma and currently in Phase II for Migraine.
pharmaphorum
JANUARY 28, 2021
New findings from a digital study have challenged the belief that stress is a significant factor in triggering migraine attacks. At the same time 76% of the total number of migraine attacks were associated with either flat or decreasing levels of perceived stress.

Pharmacy Times
JANUARY 4, 2023
There is a growing body of evidence that foods, substances, and additives in vitamins and supplements can be a trigger for migraine headaches.

Pharmaceutical Technology
JULY 19, 2022
AbbVie has filed a Marketing Authorization Application (MAA) with the European Medicines Agency (EMA) for its atogepant for prophylaxis of migraine in adults who experience a minimum of four migraine days each month. An oral CGRP receptor antagonist (gepant), atogepant is developed as a preventive migraine treatment.

pharmaphorum
NOVEMBER 18, 2020
UK cost-effectiveness agency NICE has said that Eli Lilly’s Emgality can be made available through the NHS for migraine prevention, the second drug in the CGRP inhibitor class to achieve that milestone.

pharmaphorum
NOVEMBER 18, 2020
UK cost-effectiveness agency NICE has said that Eli Lilly’s Emgality can be made available through the NHS for migraine prevention, the second drug in the CGRP inhibitor class to achieve that milestone.

PharmaShots
JUNE 1, 2023
Your go-to media platform for customized news ranging for multiple indications. For more information connect with us at connect@pharmashots.com

pharmaphorum
JULY 18, 2022
AbbVie has submitted its oral CGRP inhibitor atogepant for prevention of both episodic and chronic migraine in the EU as it tries to catch up with main rivals Pfizer/BioHaven and their recently-approved Vydura drug. GlobalData has said it expects Qulipta to get approval for chronic migraine prevention in 2023, helping it to sales of $1.2

pharmaphorum
NOVEMBER 15, 2021
The market for migraine treatment and prevention has been transformed by the launch of several new drugs targeting CGRP for many sufferers, but Lundbeck thinks there is still room for new approaches. People given injections of PACAP were shown to develop migraine-like headaches in another small study.

Med Ed 101
JULY 20, 2022
Valproic acid is most commonly used for the management of seizures, bipolar disorder, and migraines. There are a lot of valproic acid clinical pearls that you need to remember, and I’m going to highlight some of the most important ones […]. The post Valproic Acid Clinical Pearls appeared first on Med Ed 101.
ALiEM - Pharm Pearls
SEPTEMBER 11, 2023
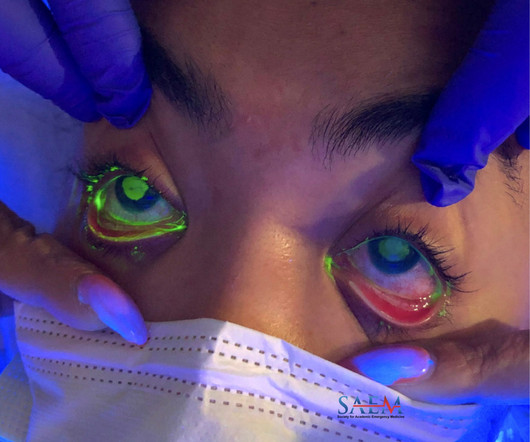
A 29-year-old female with a past medical history of migraine headaches presented to the emergency department (ED) for several hours of bilateral eye pain, redness, and decreased visual acuity. View other cases from this Clinical Image Series on ALiEM. The patient is a contact lens wearer.

pharmaphorum
NOVEMBER 10, 2021
billion deal to claim rights outside the US to Biohaven’s Nurtec ODT, an oral therapy for migraine. Under the terms of the deal, Biohaven would be responsible for further clinical development of Nurtec ODT and the two partners will cooperate on securing approval for the product in ex-US markets.
Pharmacy Times
DECEMBER 7, 2023
Amanda Hickman, PharmD, MPH, MSCS, discusses the clinical assessment to lower migraines (CALM) in an HSSP setting.

PharmaShots
JUNE 23, 2023
Gilead Receives EMA’s CHMP Positive Opinion of Trodelvy (sacituzumab govitecan) for Pre-Treated HR+/HER2- Metastatic Breast Cancer Date: June 23, 2023 | Tags: Gilead, Trodelvy, sacituzumab govitecan, HR+/HER2- Metastatic Breast Cancer, Regulatory, EMA, CHMP, Positive Opinion Sarepta Therapeutics’ Elevidys Receives the US FDA’s Accelerated (..)

pharmaphorum
MARCH 14, 2022
SMi’s 22nd Pain Therapeutics Conference will cover the leading advances in pain therapeutics, exploring late development clinical trials, novel treatments for chronic conditions, technology collaboration treatments, using VR and Medical Devices that carry out non-invasive nerve stimulation. Analgesic pre-clinical development.

Pharmaceutical Technology
JUNE 10, 2023
RT-002 is under clinical development by Revance Therapeutics and currently in Phase II for Upper Limb Muscle Spasticity. Attributes of the drug, company and its clinical trials play a fundamental role in drug-specific PTSR and likelihood of approval. It is administered by intramuscular route and a unspecified indication.

Pharmaceutical Technology
JUNE 10, 2023
RT-002 is under clinical development by Revance Therapeutics and currently in Phase II for Upper Limb Muscle Spasticity. Attributes of the drug, company and its clinical trials play a fundamental role in drug-specific PTSR and likelihood of approval. It is administered by intramuscular route and a unspecified indication.

PharmaShots
APRIL 21, 2023
Oblato Reports the First Patient Enrolment of OKN-007 in the P-I Clinical Trial for Recurrent High-Grade Glioma Date: Apr 21, 2023 | Tags: Oblato, OKN-007, Recurrent High-Grade Glioma, Regulatory, Henry Ford Health System CARsgen's CT041 Receives the US FDA’s IND Clearance for the Postoperative Adjuvant Therapy of Pancreatic Cancer Date: (..)

Med Ed 101
FEBRUARY 21, 2018
Valproic acid for migraines isn’t generally the first line prophylactic agent as there are a few other agents that can be utilized. When using valproic acid for migraines there are definitely a few things to think about clinically. The post Valproic Acid for Migraines appeared first on Med Ed 101.

PharmaShots
FEBRUARY 17, 2023
Difficile Infection Date: Feb 15, 2023 | Tags: Seres Therapeutics, SER-109, Recurrent C. to go Public via Austin Biosciences SPAC Merger for ~$166.3M to go Public via Austin Biosciences SPAC Merger for ~$166.3M

Express Pharma
MARCH 21, 2023
In September 2022, Zydus had announced positive Phase 2 proof-of-concept (POC) study in CAPS patients, and the publication of Phase 1 study results in Clinical Pharmacology in Drug Development, supporting the advancement of ZYIL1 into pivotal clinical trials in CAPS patients. There were no Serious Adverse Events (SAE’s) observed.”
pharmaphorum
NOVEMBER 11, 2022
The lack of diversity in clinical trials has been a topic of debate for decades, but was thrust into the spotlight as the impact of the pandemic on poorer, less educated and ethnically diverse populations became even more apparent. The post IQVIA’s report card for clinical trial diversity: must do better appeared first on.

European Pharmaceutical Review
JULY 18, 2022
Nebido is typically administered by a physician every 10-14 weeks for treatment of male hypogonadism or testosterone deficiency, It can also be used to treat clinical symptoms such as regression of secondary sexual characteristics, change in body composition, asthenia, reduced libido or erectile dysfunction.

pharmaphorum
SEPTEMBER 28, 2021
It also missed a key secondary measure, the Clinical Global Impression of Improvement (CGI-I) score, but Biohaven said safety data was consistent with the overall profile of verdiperstat from prior clinical trial experience. While prospects for the drug have taken a dive.
European Pharmaceutical Review
JANUARY 11, 2024
To overcome these challenges, there have been efforts in creating more accurate bedside testing, such as eg, Quantitative Sensory Testing, and more emphasis is put on early clinical translational studies. What is the present clinical landscape for pain management medicines, especially chronic pain? References Cruccu G, Truini A.

Med Ed 101
SEPTEMBER 19, 2018
I’ll give you 5 important clinical pearls below when considering using the tricyclic antidepressants. Using tricyclic antidepressants (TCA) for fibromyalgia or other pain syndromes like neuropathy or possibly even migraine is sometimes useful. […]. Know Thy Indication.

Pharmaceutical Technology
NOVEMBER 29, 2022
Researchers are also interested in ketamine’s role for the treatment of migraines. Citing his clinical experience, Dr. Eric Schwenk, professor of anesthesiology and orthopedic surgery at Thomas Jefferson University in Philadelphia, says that there are many people with migraine who would benefit from ketamine.
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?

Let's personalize your content