Top 5 takeaways from international hybrid and virtual meeting guidance for pharma
pharmaphorum
MAY 25, 2022
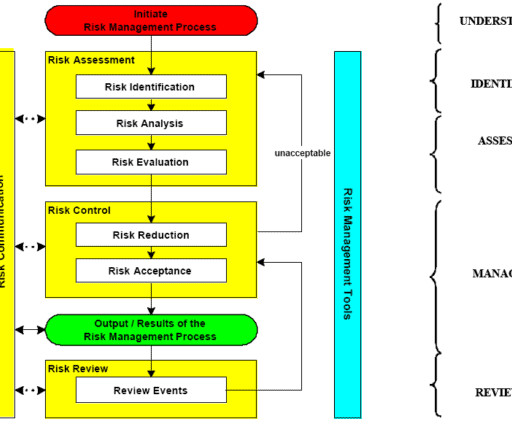
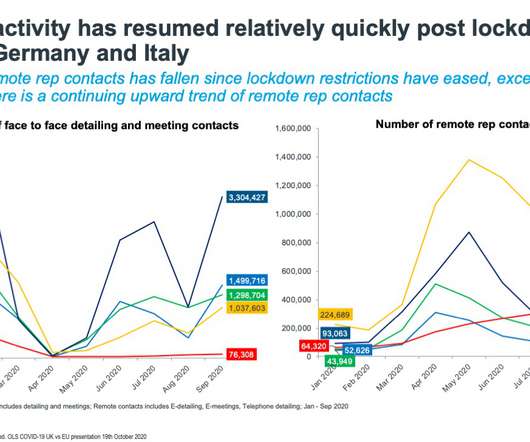
When events moved online, societies and associations recognised the wider access afforded by “virtual”, and now, as the world slowly gets back on track, many have opted for a hybrid model that offers the best of both. Here, we take a look at the top five takeaways from the document: 1. The same cannot be said for online events.














































Let's personalize your content