Quality Assurance Job Package
PharmaState Academy
MARCH 28, 2024
QUALITY ASSURANCE FULL JOB PACKAGE Start your Quality Assurance journey with our curated job package! Membership is a package in which you get lot of learning courses at much economical price & most of the time payment option of installment is available. Gain essential skills for QA roles and launch your career today!


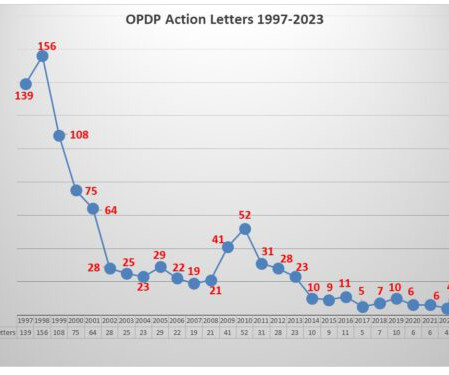











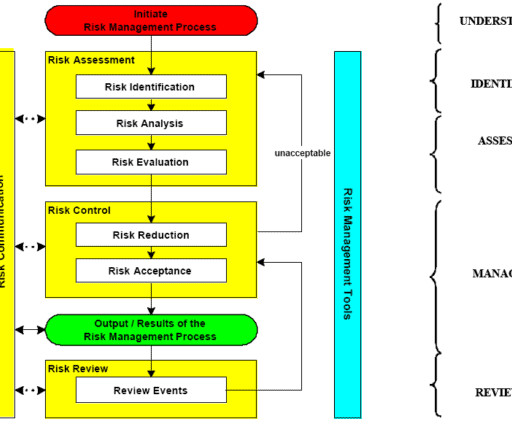






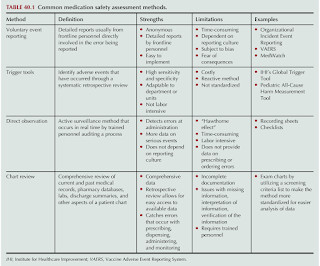

















Let's personalize your content