Leading clinical packaging companies in contract marketing
Pharmaceutical Technology
SEPTEMBER 20, 2022
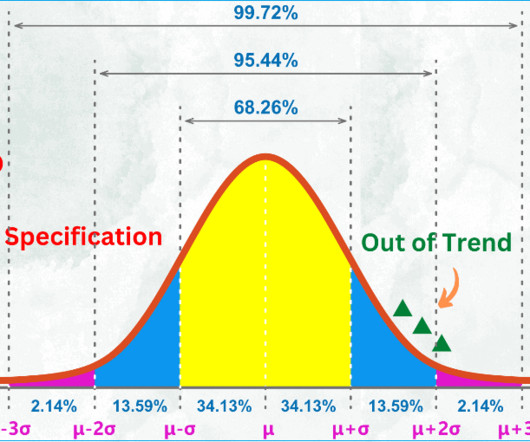
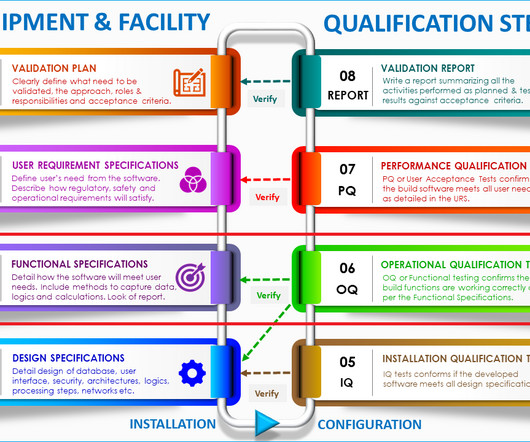
Clinical packaging and labelling follow stringently controlled procedures and high-standard quality control measures to assure the safety and functionality of investigational medicinal products, during their storage, distribution, and use. Finding the best clinical trial packaging services providers.


































Let's personalize your content