SCOTUS’ abortion pill mifepristone case is really about the FDA
STAT
MARCH 25, 2024
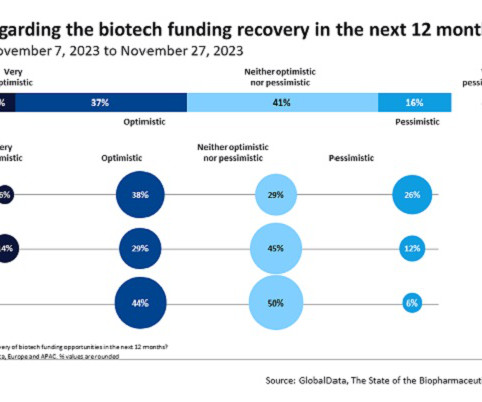
WASHINGTON — The Supreme Court on Tuesday will hear opening arguments in an abortion medication case that pharmaceutical companies warn could upend the industry and paralyze new drug development.











































Let's personalize your content