STAT Wunderkinds discuss high-impact research, resilience, and social justice
STAT
APRIL 28, 2023
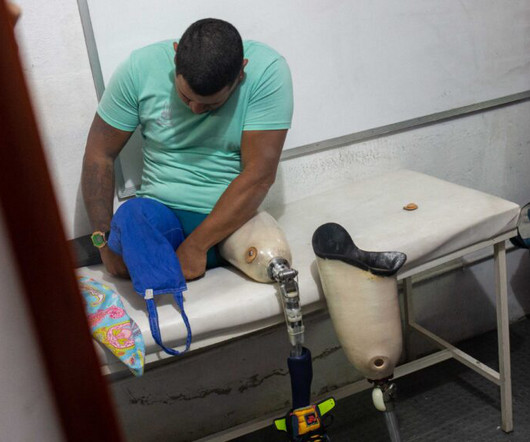
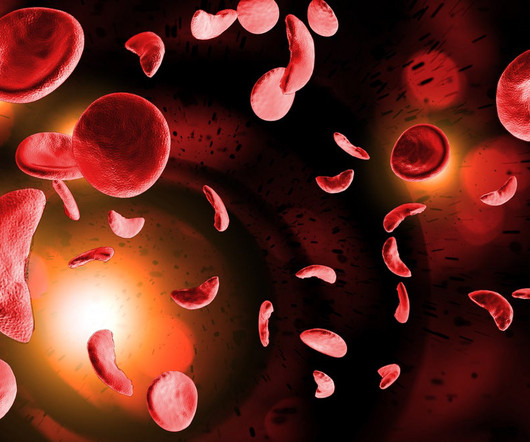
Elenoe “Crew” Smith used to faint at the sight of blood. Still, she knew from a very young age that she wanted to help people living with sickle cell disease. Now, as a research director at Vertex Pharmaceuticals, she is working on potential sickle cell medicines. Smith was in STAT’s 2017 class of Wunderkinds , an annual selection of standout researchers who are launching their careers.



































Let's personalize your content