Opinion: The amoxicillin shortage continues to force pediatricians and families to scramble
STAT
MAY 8, 2023
“S o, you don’t have it in liquid form?” I asked. The answer, as usual, was no. I hung up the phone and sighed.
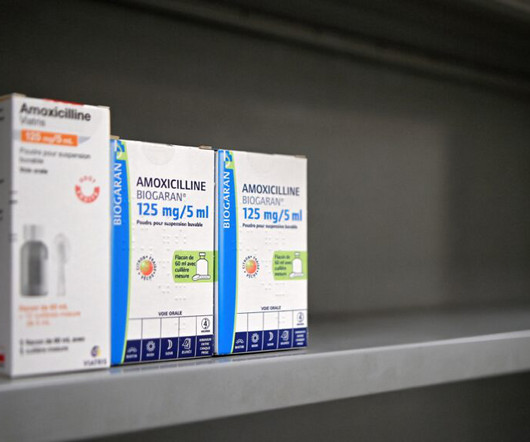
STAT
MAY 8, 2023
“S o, you don’t have it in liquid form?” I asked. The answer, as usual, was no. I hung up the phone and sighed.

PharmaVoice
MAY 8, 2023
The nonprofit generics maker still sees a need for low-cost insulin despite recent moves by major drugmakers to slash the prices of their popular products.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.
STAT
MAY 8, 2023
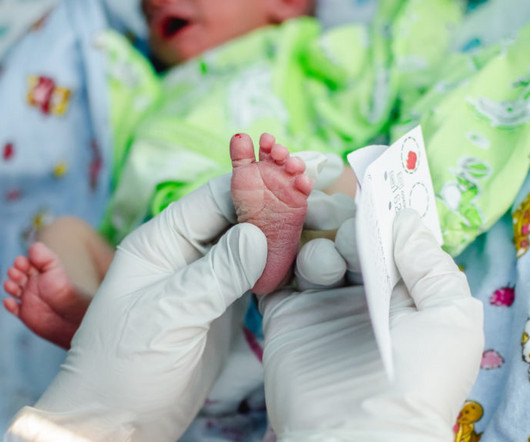
Plummeting costs of DNA sequencing technologies are injecting urgency into the longstanding debate over whether to dive deeper into the genomes of more infants — even apparently healthy ones. Experts are divided about how helpful DNA sequencing data really are. The tests often identify mutations that raise someone’s risk of developing a condition, but don’t necessarily cause the disease.

LifeProNow
MAY 8, 2023
May 09, 2023: “AstraZeneca’s Farxiga (dapagliflozin) has been approved in the US to reduce the risk of cardiovascular (CV) death, hospitalisation for heart failure (hHF) and urgent heart failure (HF) visits in adults with HF. The approval by the Food and Drug Administration (FDA) was based on positive results from the DELIVER Phase III trial. 1 Farxiga was previously approved in the US for adults with HF with reduced ejection fraction (HFrEF).

Speaker: Chris Antypas and Josh Halladay
Access to limited distribution drugs and payer contracts are key to pharmacy expansion. But how do you prepare your operations to take the next step? Meaningful data: Collect and share clinical data regarding outcomes, utilization, and more Reporting: Limited distribution models require efficient tracking and reporting systems Workflows: Align workflows with specific pharma and payer contractual requirements For in-depth, expert insights on pharmacy expansion, watch this webinar from Inovalon.
STAT
MAY 8, 2023
EQRx, a company that aimed to lower drug prices by introducing inexpensive me-too medicines, said Tuesday that it has abandoned that plan and is laying off nearly 60% of its workforce. The company’s original business plan was to create new medicines in the same categories as high-priced cancer and specialty drugs and to sell them at lower prices through a “global buyers club” that included insurers and hospitals.
LifeProNow
MAY 8, 2023
May 05, 2023: “Bristol Myers Squibb announced that the European Commission (EC) has granted approval for Breyanzi (lisocabtagene maraleucel; liso-cel), a CD19-directed chimeric antigen receptor (CAR) T cell therapy, for the treatment of adult patients with diffuse large B-cell lymphoma (DLBCL), high grade B-cell lymphoma (HGBCL), primary mediastinal large B-cell lymphoma (PMBCL) and follicular lymphoma grade 3B (FL3B), who relapsed within 12 months from completion of, or are refractory t
Pharmacist Digest brings together the best content for pharmacists from the widest variety of industry thought leaders.

PharmaVoice
MAY 8, 2023
Although approvals are off to a slow start in oncology, the agency has been living in the fast lane for neurological disorders and more.

STAT
MAY 8, 2023
Amid rising demand for drugs like Wegovy , Ozempic, and Mounjaro that can lead to significant weight loss , some obesity experts are concerned about the drugs’ costs — both to patients’ finances and to their health. Speaking at a panel on new obesity treatments at the STAT Breakthrough Summit in San Francisco on Thursday, one expert cited a large clinical trial for Wegovy that showed that about 40% of the weight participants lost was lean mass.

LifeProNow
MAY 8, 2023
May 8, 2023: “ Koselugo (selumetinib) has been approved in China for the treatment of symptomatic, inoperable plexiform neurofibromas (PN) in paediatric patients with neurofibromatosis type 1 (NF1) aged three years and above. The approval by the National Medical Products Administration (NMPA) in China was based on positive results from the SPRINT Stratum 1 trial sponsored by the National Institutes of Health’s National Cancer Institute (NCI) Cancer Therapy Evaluation Program (CTEP).

STAT
MAY 8, 2023
For the first time, the U.S. government will pay for a large study measuring whether overdoses can be prevented by so-called safe injection sites, places where people can use heroin and other illegal drugs and be revived if they take too much. The grant provides more than $5 million over four years to New York University and Brown University to study two sites in New York City and one opening next year in Providence, R.I.

Advertisement
Are you still using workarounds to manage your daily operations? To achieve peak performance, it's time to explore other options for specialty and infusion pharmacy software. Streamline pharmacy operations and improve clinical performance with automated processing, real-time data exchange, and electronic decision support. Download this helpful infographic to: Drive efficiency and patient adherence from referral receipt to delivery and ongoing care – all with our Pharmacy Cloud.

BioPharma Dive
MAY 8, 2023
Launched in 2020 with a "radical" vision, EQRx ran into roadblocks that stymied its efforts to develop new medicines and undercut competitors on price.

STAT
MAY 8, 2023
If you have been looking for a sense of pandemic closure, the World Health Organization’s declaration Friday that it was ending the Covid global health emergency was about as close to it as you are likely to get. The reality is that although battlefield metaphors are often employed to describe humankind’s struggle with the SARS-CoV-2 virus, there will be no 11th hour of the 11th day of the 11th month-like moment signaling that an armistice has been achieved.

BioPharma Dive
MAY 8, 2023
The company agreed to buy Cyclica and Valence after winnowing down a list of more than 100 potential takeover targets, its CEO told BioPharma Dive.

STAT
MAY 8, 2023
NEW YORK — The Bronx auditorium was bustling with pregnant people, and Detective Fred Washington of the police department’s community affairs bureau had a promise. “If anyone goes into labor, NYPD is here to help!” he shouted to the hundreds of people who had come from around the Bronx to the community baby shower to receive donated diapers, pacifiers, and children’s clothing.

BioPharma Dive
MAY 8, 2023
Although there is currently no cure, there is great promise in the fight against lupus. Real-world data and artificial intelligence are both critical to the future of clinical research.

STAT
MAY 8, 2023
For season 2 of “Color Code,” we’re zooming into the birthplace of American suburbs and the place where I grew up: Long Island, N.Y. Suburban communities in the U.S. have a reputation for being largely white, wealthy, and healthy, but the reality is much more complex. People from a wide swath of racial, ethnic, and socioeconomic backgrounds call suburbia home.

BioPharma Dive
MAY 8, 2023
The pharmaceutical CDMO said it found “significant” issues with its forecasts over the past year, compounding manufacturing problems at three of its plants.

STAT
MAY 8, 2023
A long-running flurry of hospital and medical group acquisitions in Pennsylvania — especially among the giants UPMC and Highmark Health — forced Geisinger to make a bigger move of its own and to sell to Kaiser Permanente. That’s according to Gail Wilensky, who has been on the board of Geisinger since 2010. Wilensky is an economist by training, and also is well-known in health policy circles.
Pharmaceutical Technology
MAY 8, 2023
The pharmaceutical industry continues to be a hotbed of innovation, with activity driven by the evolution of new treatment paradigms, and the gravity of unmet needs, as well as the growing importance of technologies such as pharmacogenomics, digital therapeutics, and artificial intelligence. In the last three years alone, there have been over 633,000 patents filed and granted in the pharmaceutical industry, according to GlobalData’s report on Immuno-oncology in Pharmaceuticals: Microbio t a rest

STAT
MAY 8, 2023
When Herlda Senhouse looks back — way back — in time, she vividly remembers the smells — the sour tang of the beer she siphoned into bottles on her first job while still in grammar school in the early 1920s and the pervasive rotten egg odor from the paper mill near her childhood home in West Virginia. Sitting at the dining room table in her Wellesley, Mass., apartment, Senhouse, a slight woman with a smooth-as-honey Southern accent, is quick to summon me

FDA Law Blog: Biosimilars
MAY 8, 2023
By Lisa M. Baumhardt, Senior Medical Device Regulation Expert & Philip Won & Gail H. Javitt — FDA recently published a long-awaited draft guidance aimed at reducing the need for prior FDA authorization of modifications to artificial intelligence/machine learning (AI/ML)-enabled device software functions (ML-DSFs). The draft guidance, “Marketing Submission Recommendations for a Predetermined Change Control Plan for Artificial Intelligence/Machine Learning (AI/ML)-Enabled Device Software

STAT
MAY 8, 2023
Good morning, everyone, and welcome to another working week. We hope the weekend respite was refreshing and inspiring, because that oh-so familiar routine of online meetings, phone calls, and deadlines has predictably returned. But you knew this would happen, yes? So to cope, we are firing up the trusty coffee kettle to brew some needed cups of delicious stimulation.
Pharmaceutical Technology
MAY 8, 2023
The pharmaceutical industry continues to be a hotbed of innovation, with activity driven by the evolution of new treatment paradigms as well as the growing importance of technologies such as pharmacogenomics, digital therapeutics, and artificial intelligence. In the last three years alone, there have been over 633,000 patents filed and granted in the pharmaceutical industry, according to GlobalData’s report on Innovation in Pharmaceuticals: Anti-influenza antibody compositions.

STAT
MAY 8, 2023
You’re reading the web version of Health Care Inc., STAT’s weekly newsletter following the flow of money in medicine. Sign up to get it in your inbox every Monday. Gail Wilensky on Geisinger’s sale to Kaiser No deal is bigger in health care right now than Kaiser Permanente’s takeover of Geisinger. It came out of nowhere and will lead to the formation of a new national mega-system of integrated health systems.

Pharmaceutical Technology
MAY 8, 2023
Ei s ai and Bliss Biopharmaceutical have entered into a joint development agreement for antibody-drug conjugate (ADC), BB-1701, to treat cancers. This deal also includes option rights for a strategic collaboration. The collaboration will help in further development of BB-1701 across the world and advance the ADC towards late stage of development. Currently, BB-1701 is being evaluated in Phase I/II trials in China and the US.

BioPharma Dive
MAY 8, 2023
Providing accurate information to protect patients from misinformation is a key goal for pharmaceutical companies, and print media can be a crucial part of the solution.

Pharmaceutical Business Review
MAY 8, 2023
The inspection will allow the company to commence commercial operations at the three-storey, 84,000ft² manufacturing facility. Nexus Pharmaceuticals stated that the regulatory approval process validates facilities’ compliance with stringent quality and safety standards. Equipped with advanced isolator technology, the Pleasant Prairie facility adheres to the highest current good manufacturing practice (CGMP) standards.

PhRMA
MAY 8, 2023
Indiana just joined West Virginia and Arkansas in delivering an important win for Hoosiers who are struggling to afford their medicines. This week, Governor Holcomb signed into law Senate Bill 8 recently passed by the Indiana legislature. This new law will ensure that Indiana patients aren’t paying more for their medicines than their health insurance company or the middlemen known as pharmacy benefit managers (PBMs).

Express Pharma
MAY 8, 2023
Eisai Co has entered into a joint development agreement with Bliss Biopharmaceutical (Hangzhou) for BB-1701, an antibody-drug conjugate (ADC) with option rights for strategic collaboration. BB-1701 is an ADC that is composed of Eisai’s in-house developed anticancer agent eribulin, and anti-HER2 antibody using a linker, and is expected to have anti-tumour effects on breast, lung and other solid tumours that express HER2.

PhRMA
MAY 8, 2023
Researching and developing new medicines for children is a priority for the biopharmaceutical industry. Despite the scientific complexity and challenges inherent to developing treatments for our youngest patients, there has never been more progress in pediatric health outcomes. The progress we are seeing in pediatric health outcomes is in part due to the advancement in medicines that have been specifically studied in and approved for use in children.
European Pharmaceutical Review
MAY 8, 2023
A marketing authorisation for PRX-102 (pegunigalsidase alfa) , the first Poly(ethylene glycol) (PEG)ylated enzyme for Fabry disease, has been granted by the European Commission (EC). The approval follows the European Medicines Agency (EMA) human medicines committee’s recommendation of the enzyme replacement therapy (ERT) in February 2023. CHMP recommends first pegylated enzyme for Fabry disease… The PEGylated enzyme replacement therapy Chiesi Global Rare Diseases and Protalix BioTherapeutics, In

Express Pharma
MAY 8, 2023
IOL Chemicals and Pharmaceuticals has received the European Directorate for the Quality of Medicines & HealthCare’s (EDQM) Certificate of Suitability (CEP) to export paracetamol to the European market. Paracetamol is utilised commonly in medication prescribed for pain relief and to treat fever. The certification issued by the EDQM verifies the compliance of pharma substances and with this backing, IOL will now be able to export paracetamol to the European continent.

World Pharma News
MAY 8, 2023
Roche (SIX: RO, ROG; OTCQX: RHHBY) announced the launch of the Institute of Human Biology (IHB) focussing on advancing research in the field of human model systems such as organoids. Leveraging human model systems, the institute aims to accelerate drug discovery and development by improving the understanding of how organs function and how diseases develop.

Express Pharma
MAY 8, 2023
Baxter International has signed a definitive agreement to divest its BioPharma Solutions (BPS) business to Advent International (Advent) and Warburg Pincus. Under the terms of the definitive agreement, Baxter will receive $4.25 billion in cash, subject to certain closing adjustments, with net after-tax proceeds currently estimated to be approximately $3.4 billion.
Pharmaceutical Technology
MAY 8, 2023
The pharmaceutical industry continues to be a hotbed of innovation, with activity driven by the evolution of new treatment paradigms, and the gravity of unmet needs, as well as the growing importance of technologies such as pharmacogenomics, digital therapeutics, and artificial intelligence. In the last three years alone, there have been over 633,000 patents filed and granted in the pharmaceutical industry, according to GlobalData’s report on Innovation in Pharmaceuticals: Anti-viral antigen-bas
Let's personalize your content