EU backs approval of Moderna’s COVID-19 vaccine in 12-17 year-olds
Pharma Times
JULY 27, 2021
Study in adolescents met it primary endpoint, successfully bridging immune responses to those observed in an efficacy study in adults
Pharma Times
JULY 27, 2021
Study in adolescents met it primary endpoint, successfully bridging immune responses to those observed in an efficacy study in adults

pharmaphorum
JULY 23, 2021
Could virtual reality tools be effective in helping patients ‘unlearn’ their chronic pain? p harmaphorum speaks to Professor Christopher Eccleston from the University of Bath’s Centre of Pain Research to find out how digital therapeutics are shaping the future of pain management. A digital software developed by Finnish drugmaker Orion is aiming to address chronic pain conditions using virtual reality (VR) devices that provide an immersive gamified therapeutic treatment program.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

NY Times
JULY 14, 2021
The company said it had determined that daily exposure to five Neutrogena and Aveeno sprays would not cause adverse health effects, but recalled the products out of an abundance of caution.
Outsourcing Pharma
JULY 14, 2021
The two companies will work together to come up with solutions that use machine learning and artificial intelligence to help accelerate innovation in R&D.

Speaker: Chris Antypas and Josh Halladay
Access to limited distribution drugs and payer contracts are key to pharmacy expansion. But how do you prepare your operations to take the next step? Meaningful data: Collect and share clinical data regarding outcomes, utilization, and more Reporting: Limited distribution models require efficient tracking and reporting systems Workflows: Align workflows with specific pharma and payer contractual requirements For in-depth, expert insights on pharmacy expansion, watch this webinar from Inovalon.

Eye on FDA
JULY 29, 2021
In the face of a crisis situation, it is a given that the clarity and thoroughness of the communications response is key to resolving the issue and mitigating any reputational damage. Perhaps no other decision by the Food and Drug Administration has garnered as much controversy, as the recent one to authorize the accelerated approval a new treatment for Alzheimer’s.

Drug Patent Watch
JULY 16, 2021
Come join me and Wendi Lau from Abbvie as we participate in an interactive roundtable discussion on pharmaceutical portfolio management. We’ll be sharing our expertise and best practices for strategic…. The post DrugPatentWatch and Abbvie discuss Pharmaceutical Portfolio Management appeared first on DrugPatentWatch - Make Better Decisions.
Pharmacist Digest brings together the best content for pharmacists from the widest variety of industry thought leaders.

pharmaphorum
JULY 6, 2021
The UK’s biotechnology sector is going through a purple patch, attracting almost £1.6 billion ($2.2 billion) in financing in the three months to end-May – which is a record for the industry. The new figures – from the BioIndustry Association and Clarivate – were dominated by more than £1 billion in venture capital funding for UK biotech and life sciences companies, headlined by DNA sequencing company Oxford Nanopore’s £195 million raise in May that was just shy of the £205 million re

NY Times
JULY 5, 2021
The World Bank said the financial crisis could rank among the world’s three worst since the mid-1800s. The currency has lost more than 90 percent of its value and unemployment has skyrocketed.

Outsourcing Pharma
JULY 8, 2021
The immunotherapy firm is conducting a Phase III trial centered on an innovative approach to treating recurrent glioblastoma, an aggressive brain cancer.

Eye on FDA
JULY 19, 2021
It has been a regular feature of the blog to give a read on what FDA has been talking about, at least through the form of press releases. It is not always an easy task because the nature of FDA’s practice in this respect has evolved over time. But here is what we see looking at the first 6 months 2021, and comparing it to mid-years past. You may recall from the last posting on this topic at the beginning of the year, during 2020, FDA had a lot to say – a real lot, and that isn’

Advertisement
Are you still using workarounds to manage your daily operations? To achieve peak performance, it's time to explore other options for specialty and infusion pharmacy software. Streamline pharmacy operations and improve clinical performance with automated processing, real-time data exchange, and electronic decision support. Download this helpful infographic to: Drive efficiency and patient adherence from referral receipt to delivery and ongoing care – all with our Pharmacy Cloud.
Drug Patent Watch
JULY 22, 2021
Annual Drug Patent Expirations for LENVIMA Lenvima is a drug marketed by Eisai Inc and is included in one NDA. There are five patents protecting this drug and one Paragraph…. The post New patent for Eisai Inc drug LENVIMA appeared first on DrugPatentWatch - Make Better Decisions.

Pharma Times
JULY 13, 2021
Researchers discover presence of 'autoantibodies' in people with persistent COVID-19 symptoms
pharmaphorum
JULY 26, 2021
You’ve had your two COVID-19 jabs, but are you actually protected against infection? That’s a question that a fingerprick test launched today in the UK and Ireland could help to answer. The test – originally developed by US manufacturer Chembio Diagnostics – has been introduced into the UK and Irish markets by Guilford-based Luas Diagnostics and tests for the presence of SARS-CoV-2-targeting antibodies in the blood.
NY Times
JULY 12, 2021
Federal regulators concluded that the risk of developing the syndrome was low, and that the benefits of the vaccine still strongly outweigh it.
Outsourcing Pharma
JULY 12, 2021
Antibody responses against the SARS-CoV-2 spike protein were found in 99% of volunteers after the second dose of ReiTheraâs COVID-19 vaccine candidate, according to Phase 2 preliminary data.

Pharma in Brief
JULY 29, 2021
CADTH has convened a pan-Canadian Advisory Panel on a Framework for a Prescription Drug List (the Panel ). The Panel will provide recommendations on developing a potential pan-Canadian prescription drug list (or formulary). Stakeholder consultations are scheduled to take place in the fall and winter of 2021, leading to a final public report setting out the Panel’s non-binding recommendations in April 2022.

Drug Patent Watch
JULY 21, 2021
Annual Drug Patent Expirations for GOCOVRI Gocovri is a drug marketed by Adamas Pharma and is included in one NDA. It is available from one supplier. There are fifteen patents…. The post New patent for Adamas Pharma drug GOCOVRI appeared first on DrugPatentWatch - Make Better Decisions.

Pharma Times
JULY 7, 2021
Eli Lilly and Boehringer Ingelheim have revealed positive results from a Phase III study of Jardiance in HFpEF patients

pharmaphorum
JULY 13, 2021
Faced with a looming mental health crisis, the UK government has been urged to invest in the digitisation of service provision across the NHS in England in a new report from think tank Future Care Capital. The study’s author – FCC’s head of policy and research Dr Peter Bloomfield, argues that there has been a lack of support for mental health services for many years in the UK, and the pathways for accessing them are “convoluted, waiting lists are extensive, and outcomes are po

NY Times
JULY 1, 2021
The Pfizer and Moderna vaccines are safe and effective. Full approval from the F.D.A. will help stop the spread of Covid-19.

Outsourcing Pharma
JULY 19, 2021
A recent study indicates patients taking the cholesterol-lowering drugs prior to COVID hospitalization are far less likely to die as a result of the virus.
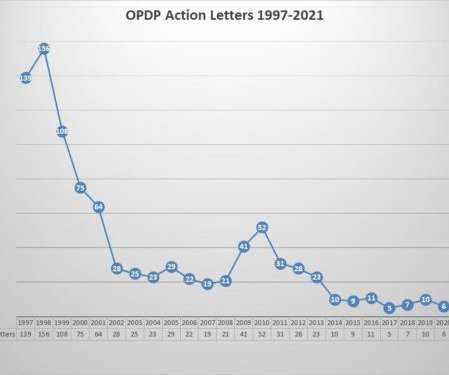
Eye on FDA
JULY 14, 2021
Upfront Author’s Note: This is the first posting in a while as I have been working to migrate the subscription service to a new provider. As such, some subscribers were lost in the process. Anyone who signed up for a subscription prior to 2019, would need to sign up again. Apologies for the inconvenience and thanks for your patience. Years ago, a single regulatory action letter issued by FDA’s Office of Prescription Drug Promotion (OPDP) would not have merited a blog posting in and o
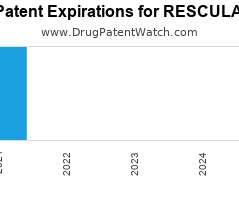
Drug Patent Watch
JULY 8, 2021
Annual Drug Patent Expirations for RESCULA Rescula is a drug marketed by Sucampo Pharma Llc and is included in one NDA. There is one patent protecting this drug and one…. The post New patent expiration for Sucampo Pharma drug RESCULA appeared first on DrugPatentWatch - Make Better Decisions.
Pharma Times
JULY 12, 2021
Vaccine effectiveness against symptomatic disease in at-risk groups is approximately 60% after one dose of either the AZ or Pfizer/BioNTech vaccines

pharmaphorum
JULY 9, 2021
Takeda’s Adam Zaeske discusses how cross-sector collaboration is shaping a brighter future for rare disease diagnosis. Diagnosis can sometimes be the most difficult part of a rare disease patient’s journey, and it’s with good reason that the process is often referred to as an ‘odyssey’ in these communities. “We’re dealing with very small populations of patients, on top of small populations of specialist physicians who are able to diagnose them,” says Adam Zaeske, formerly head of Rare Diseases,
NY Times
JULY 20, 2021
Many who received the shot may need to consider boosters, the authors said. But federal health officials do not recommend second doses.

Outsourcing Pharma
JULY 27, 2021
A recent check-in with several biopharma and CRO companies, conducted by Life Science Strategy Group, indicates adjustment to pandemic-related challenges.
Pharma Times
JULY 6, 2021
Vaccine will be evaluated in 13 HIV-negative adults aged 18-65 years old

Pharma Times
JULY 12, 2021
NICE concluded the cost-effectiveness estimates for Libmeldy are unlikely to be within the range that it would consider an effective use of NHS resources

pharmaphorum
JULY 15, 2021
The UK’s competition authority has issued its largest ever fine of more than £260 million ($360 million) to several pharma companies accused of colluding to hike the price of medicines delivered to the NHS. The Competition and Markets Authority (CMA) took the action against Auden Mckenzie and Actavis UK – now known as Accord-UK – and other companies after an investigation into the price of hydrocortisone tablets, which rose by 10,000% over a 10-year period.

pharmaphorum
JULY 6, 2021
A peptide vaccine developed by Australia’s Imugene has reduced tumour size in around half of patients with HER2-positive gastric or gastroesophageal junction (GEJ) cancer in a phase 2 trial. The interim readout from the 39-patient HERIZON trial found 50% of patients treated with the HER-Vaxx (IMU-131) vaccine on top of chemotherapy achieved a partial response or better, compared to 29% of patients given only chemo.

Pharma Times
JULY 22, 2021
Verquvo cleared for the treatment of symptomatic chronic heart failure in patients with reduced ejection fraction

pharmaphorum
JULY 26, 2021
A lack of racial diversity in clinical trials is a long-standing, well-documented problem that contributes to the stark health inequalities that have been brought into sharp focus by COVID-19. Tackling it, however, has not been easy, thanks in no small part to the reasons being as complex as they are multi-faceted. But, according to the Pharmaceutical Research and Manufacturers of America (PhRMA), the country’s biopharmaceutical companies are “committed to learning and leading” in a bid to “addr
pharmaphorum
JULY 14, 2021
AstraZeneca and Johnson & Johnson are both exploring ways to modify their COVID-19 vaccines to minimise the risk of severe blood clotting reactions that are seen – albeit rarely – in some people receiving the jabs. At the same time, the UK’s National Institute for Health and Care excellence (NICE) is developing guidance to help doctors manage the cases of vaccine-induced immune thrombocytopenia and thrombosis (VITT) and low blood platelet counts that have been seen with the COVID-19 va
Pharma Times
JULY 28, 2021
Rate of rare blood clots with low platelets after the second dose is comparable to background rates observed in unvaccinated populations
Let's personalize your content