University of Oxford begins human trials of Ebola vaccine
Pharma Times
NOVEMBER 11, 2021
ChAdOxl biEBOV is being tested for safety and immunogenicity, and may protect against multiple species of the virus
Pharma Times
NOVEMBER 11, 2021
ChAdOxl biEBOV is being tested for safety and immunogenicity, and may protect against multiple species of the virus
pharmaphorum
NOVEMBER 12, 2021
Researchers in the US have developed an artificial intelligence-based tool that is able to predict COVID-19 symptoms and suggest which FDA-approved drugs might be used to treat patients. The MOATAI-VIR algorithm, developed by scientists at Emory University and Georgia Tech, was put through its paces in a study that showed it was able to predict 24 out of 26 clinical manifestations of COVID-19, including acute respiratory distress, blood clotting issues, cytokine storms, brain fog, and loss of sm
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.
Drug Patent Watch
NOVEMBER 10, 2021
Annual Drug Patent Expirations for ARISTADA+INITIO+KIT Aristada Initio Kit is a drug marketed by Alkermes Inc and is included in one NDA. It is available from one supplier. There are…. The post New patent for Alkermes Inc drug ARISTADA INITIO KIT appeared first on DrugPatentWatch - Make Better Decisions.

NY Times
NOVEMBER 9, 2021
Moderna’s patent application names several employees as the sole inventors of a crucial component of its coronavirus vaccine, excluding three government scientists.

Speaker: Chris Antypas and Josh Halladay
Access to limited distribution drugs and payer contracts are key to pharmacy expansion. But how do you prepare your operations to take the next step? Meaningful data: Collect and share clinical data regarding outcomes, utilization, and more Reporting: Limited distribution models require efficient tracking and reporting systems Workflows: Align workflows with specific pharma and payer contractual requirements For in-depth, expert insights on pharmacy expansion, watch this webinar from Inovalon.
Pharma Times
NOVEMBER 12, 2021
Researchers have said that next generation vaccines for COVID-19 should aim to induce a response against ‘replication proteins’
pharmaphorum
NOVEMBER 8, 2021
The UK government has announced £248 million in spending over the next year for a project to make it easier for patient test results and scans to be shared between hospitals, labs and GP surgeries. The aim is to “digitise diagnostics care” across the NHS, reducing the time it takes to diagnose health problems and get treatment started earlier, helping to address the massive backlog in care caused by the pandemic, according to a Department of Health and Social Care (DHSC) statement.
Pharmacist Digest brings together the best content for pharmacists from the widest variety of industry thought leaders.

Pharma Marketing Network
NOVEMBER 8, 2021
November 3, 2021 – Tune in with the Pharma Marketing Network for an exciting conversation with R.J. Lewis and David Hunt, CEO of The Considered, as he shares how his new independent agency, The Considered, about how they are breaking the rules in pharma advertising. David discusses and answers questions around: How The Considered is different. What launched the conception of the agency?
Pharma Times
NOVEMBER 10, 2021
Janssen's Ponvory accepted for use within NHS Scotland for adults RRMS patients

pharmaphorum
NOVEMBER 9, 2021
Scotland has given the green light to AstraZeneca’s Tagrisso for restricted use to treat adult patients with non-small cell lung cancer (NSCLC), making it the first country in the world to approve the routine use of the therapy in this setting. The Scottish Medicines Consortium (SMC) has approved Tagrisso (osimertinib) for use within NHS Scotland as a monotherapy for adjuvant treatment after tumour removal in adult patients with stage IB-IIIA NSCLC whose tumours have epidermal growth factor (EGF
Outsourcing Pharma
NOVEMBER 12, 2021
Johnson & Johnson today announced its intent to separate its Consumer Health business into a new publicly traded company: allowing J&J to put a sharpened focus on pharmaceuticals and medical devices.

Advertisement
Are you still using workarounds to manage your daily operations? To achieve peak performance, it's time to explore other options for specialty and infusion pharmacy software. Streamline pharmacy operations and improve clinical performance with automated processing, real-time data exchange, and electronic decision support. Download this helpful infographic to: Drive efficiency and patient adherence from referral receipt to delivery and ongoing care – all with our Pharmacy Cloud.

Drug Channels
NOVEMBER 9, 2021
The Food & Drug Administration (FDA) recently approved the first interchangeable biosimilar insulin product: the insulin glargine-yfgn injection from Viatris. Read the FDA’s press release. Alas, I’m sad to report that the warped incentives baked into the U.S. drug channel will limit the impact of this impressive breakthrough. Viatris is being forced to launch both a high-priced and a low-priced version of the biosimilar.
Pharma Times
NOVEMBER 8, 2021
Daprodustat is an investigational oral hypoxia-inducible factor prolyl hydroxylase inhibitor in development for anaemia due to chronic kidney disease

pharmaphorum
NOVEMBER 12, 2021
The EMA’s human medicines committee has recommended approval of Roche’s Ronapreve and Celltrion’s Regkirona for use in COVID-19, the first antibody-based therapies for coronavirus to be backed for full approval in the EU. Ronapreve (casirivimab and imdevimab) – developed and distributed by Regeneron as REGN-COV in the US – has been recommended by the CHMP for the treatment and prevention of COVID-19 in people aged 12 years and older weighing at least 40kg.

Outsourcing Pharma
NOVEMBER 11, 2021
During our November 17 webinar Patient-centric solutions, a group of professionals representing a range of perspectives will share ideas and innovations.

Drug Channels
NOVEMBER 8, 2021
Informa Connect’s Trade & Channel Strategies. Delivered as a Hybrid Event In-Person: December 13-14, 2021, Hilton Philadelphia at Penn’s Landing, Philadelphia, PA Virtual: December 16-17, 2021 [link]. The full agenda for this year’s Trade and Channel Strategies conference is available now! Year after year, the life science industry marks Informa Connect’s Trade and Channel Strategies as the “go-to” event for trade, channel, market access, account strategy and brand professionals.

Pharma Times
NOVEMBER 9, 2021
iiCON’s lead partner LSTM will independently validate CN Bio’s novel lung and lung-liver models

pharmaphorum
NOVEMBER 10, 2021
Tackling antimicrobial resistance (AMR) requires a one health approach that joins the dots between the human, animal, and environmental drivers, consequences, and data. AMR threatens to undermine “almost a century of health gains” and marshalling a response to such a problem “requires cooperation at many levels”. That’s according to a newly published report from the US National Academies of Sciences, Engineering, and Medicine, which calls for a One Health approach that recognises the scale of th

Outsourcing Pharma
NOVEMBER 8, 2021
The decentralized tech specialist has acquired Digital Artefacts, a research tech firm focused on cognitive, behavioral, and physiological data capture.

NY Times
NOVEMBER 12, 2021
The 135-year-old company announced plans to divide itself into a consumer products business and a medical company.
Pharma Times
NOVEMBER 9, 2021
Funding proceeds will be used to support ongoing trials of Gyroscopes investigational gene therapy GT005
pharmaphorum
NOVEMBER 11, 2021
Shares in France’s Valneva leaped after it got an EU order to supply 60 million doses of its COVID-19 vaccine VLA2001 over the next two years, including 27 million next year. The deal for the adjuvanted, inactivated SARS-CoV-2 vaccine still depends on authorisation of the jab by the European Medicines Agency (EMA) which is due to start a rolling review of VLA2001 shortly, but Valneva thinks it could start delivering supplies to the EU in April next year.

Outsourcing Pharma
NOVEMBER 10, 2021
According to the market intelligence firm, automation appeals to drug packaging and manufacturing firms, thanks to their productivity-boosting potential.
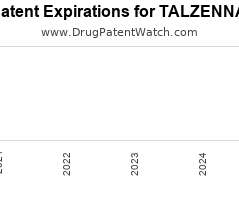
Drug Patent Watch
NOVEMBER 12, 2021
Annual Drug Patent Expirations for TALZENNA Talzenna is a drug marketed by Pfizer and is included in one NDA. It is available from two suppliers. There are five patents protecting…. The post New patent for Pfizer drug TALZENNA appeared first on DrugPatentWatch - Make Better Decisions.

Pharma Times
NOVEMBER 12, 2021
The draft guidance has been published following an evaluation of new evidence on the use of the treatment

pharmaphorum
NOVEMBER 10, 2021
After being hailed as a triumph of public-private drug development, Moderna’s partnership with the US government on its COVID-19 vaccine looks like it may descend into acrimony. That is according to a New York Times report , which says Moderna is now in dispute with the National Institutes of Health (NIH) after it filed a US patent in the US that claims the company’s scientists invented the vaccine on their own.

Pharmacist Money Blog
NOVEMBER 10, 2021
In today’s post, I will discuss five steps you can follow to pursue financial freedom also known as financial independence. 1. Pay off your debts Why do you have to pay off your debts? You might ask yourself, don’t you need debt to get rich? How can you go anywhere without getting into debt? Yes while it is true that The post 5 Steps to Financial Freedom appeared first on Pharmacist Money Blog.
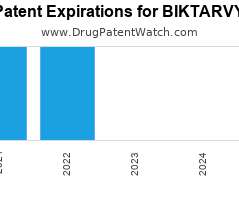
Drug Patent Watch
NOVEMBER 12, 2021
Annual Drug Patent Expirations for BIKTARVY Biktarvy is a drug marketed by Gilead Sciences Inc and is included in one NDA. It is available from two suppliers. There are ten…. The post New patent for Gilead Sciences drug BIKTARVY appeared first on DrugPatentWatch - Make Better Decisions.

Pharma Times
NOVEMBER 11, 2021
Olumiant maintained a consistent safety profile in a long-term analysis of patients with rheumatoid arthritis

pharmaphorum
NOVEMBER 10, 2021
Germany’s vaccination advisory committee has recommended that people aged under 30 should only be offered Pfizer/BioNTech’s COVID-19 vaccine Comirnaty, saying it seems less likely to cause heart inflammation than Moderna’s rival shot Spikevax. The draft guidance from the Robert Koch Institute’s STIKO committee also says that pregnant women, regardless of their age, should get the Pfizer/BioNTech vaccine.

Pharma Mirror
NOVEMBER 10, 2021
Milan, CPhI Worldwide – hosted in-person at Fiera Milano, Italy (9-11 November, 2021) – opens its doors to international pharma for the first time in two years asindustry confidence surges to record highs according to executives in the CPhI Annual Survey. The survey insights are published as part of the CPhI Annual Report – which is launched each year at CPhI Worldwide – and compile the thoughts of over 370 executives from more than 30 countries.
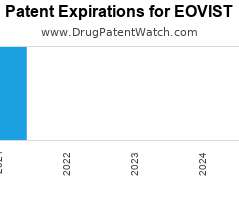
Drug Patent Watch
NOVEMBER 12, 2021
Annual Drug Patent Expirations for EOVIST Eovist is a drug marketed by Bayer Hlthcare and is included in one NDA. It is available from one supplier. There is one patent…. The post New patent expiration for Bayer Hlthcare drug EOVIST appeared first on DrugPatentWatch - Make Better Decisions.

Pharma Times
NOVEMBER 11, 2021
Decision follows the UK government’s termination of their deal with Valneva in September

pharmaphorum
NOVEMBER 11, 2021
KNUTSFORD, United Kingdom — Fishawack Health welcomes FIDE, an independent organization providing its clients with insight from clinical key opinion leaders in inflammatory dermatology. [Image: From left, FIDE Directors Richard GB Langley, MD; Richard Warren, MD, PhD; Kenneth B Gordon, MD; Bruce E Strober, MD, PhD]. November 10, 2021, UK. Fishawack Health, the leading commercialization partner for the life science industry, welcomes FIDE to its group—an independent expert-led consultancy specia

Pharma Mirror
NOVEMBER 10, 2021
Milan, Ahead of the world’s largest pharma event – CPhI Worldwide taking place in Milan, Italy (9-11 November, 2021) – the third part of the CPhI Annual Report is released with key findings for biologics manufacturing, biotechs and CDMOs. CPhI experts – Dawn Ecker, Managing Director of bioTRAK Database Services at BDO and Fiona Barry, Editor at GlobalData PharmSource – look ahead to 2025 to predict the future demand for biologicals by volume, as well as the total capacity available and, the cons
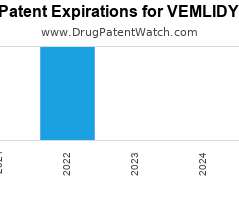
Drug Patent Watch
NOVEMBER 10, 2021
Annual Drug Patent Expirations for VEMLIDY Vemlidy is a drug marketed by Gilead Sciences Inc and is included in one NDA. It is available from one supplier. There are four…. The post New patent for Gilead Sciences drug VEMLIDY appeared first on DrugPatentWatch - Make Better Decisions.
Let's personalize your content