Why a 'fundamental shift' in regulatory submissions is on the way
PharmaVoice
JULY 11, 2022
Deloitte's senior manager of R&D and regulatory practices explains how technology and strategy must converge to smooth out the bulky drug approval process.

PharmaVoice
JULY 11, 2022
Deloitte's senior manager of R&D and regulatory practices explains how technology and strategy must converge to smooth out the bulky drug approval process.

Pharmacy Is Right For Me
JULY 11, 2022
There are so many different types of careers within the field of pharmacy—from research and drug development to pharmacy informatics! To highlight some of the more unique career settings in the industry, we’re introducing a new page on our website— Novel Pharmacy Practice Settings —where you can explore these unique career pathways. In addition to learning more about unique pathways on our new webpage, we’ll also be featuring pharmacists who work in these unique settings on our blog.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Pharmaceutical Technology
JULY 15, 2022
Insulin prices made the headlines again as California governor Gavin Newsom announced plans on 7 July for the state to manufacture low-cost insulin. In a budget change proposed in February and confirmed in May , California’s Department of Health Care Access and Information (HCAI) requested a one-time investment of $100 million for Newsom’s CalRx Biosimilar Insulin initiative.

pharmaphorum
JULY 15, 2022
Pfizer’s tyrosine kinase inhibitor Xalkori has picked up a fourth approval from the FDA, adding a new use in the treatment of a rare form of benign tumour that typically affects children and young adults. Xalkori (crizotinib) has been cleared for inflammatory myofibroblastic tumours (IMT), which appear in organs like the lung, stomach, bladder or liver.

Speaker: Chris Antypas and Josh Halladay
Access to limited distribution drugs and payer contracts are key to pharmacy expansion. But how do you prepare your operations to take the next step? Meaningful data: Collect and share clinical data regarding outcomes, utilization, and more Reporting: Limited distribution models require efficient tracking and reporting systems Workflows: Align workflows with specific pharma and payer contractual requirements For in-depth, expert insights on pharmacy expansion, watch this webinar from Inovalon.

PharmaVoice
JULY 14, 2022
The pharma giant’s ‘Depression looks like me’ campaign assembles a ‘one stop shop’ of resources to help combat high rates of severe depression in the LGBTQ community.

Pharma Times
JULY 15, 2022
Data from APHINITY study shows Roche’s Perjeta-based regimen reduces the risk of disease returning for people with HER2-positive early breast cancer
Pharmacist Digest brings together the best content for pharmacists from the widest variety of industry thought leaders.

pharmaphorum
JULY 15, 2022
Akili looks like it could bring another digital therapeutic (DTx) to market after a clinical trial backed the efficacy of its AKL-T01 in patients with the autoimmune disorder systemic lupus erythematosus (SLE). The PureTech group company – which scored the first FDA and EMA approvals for a DTx for attention-deficit hyperactivity disorder (ADHD) – is developing AKL-T01 to tackle cognitive impairments that can affect people with SLE.

PharmaVoice
JULY 13, 2022
Elizabeth Izard Apelles is finding innovative ways to champion rare disease patients through an emerging platform from Honeycomb Health.
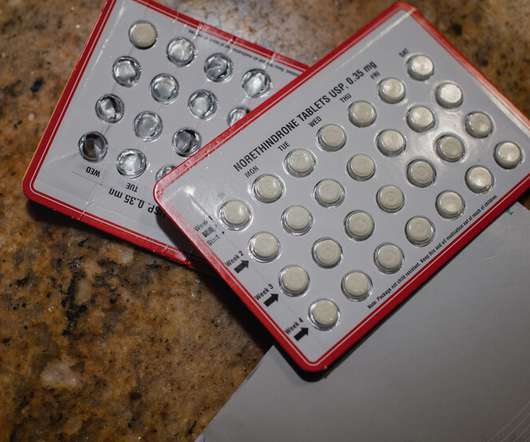
NY Times
JULY 11, 2022
The first U.S. application for sale of a nonprescription birth control pill has taken on new meaning after the Supreme Court decision ending the constitutional right to abortion.

Pharmaceutical Technology
JULY 15, 2022
Despite entering its fourth decade of availability, in vitro fertilization (IVF), a medical technique used to facilitate the conception of a baby for those facing fertility problems, remains an elusive dream for many. Artificial intelligence (AI) could change this, with many researchers looking to harness the technology to increase IVF’s reliability, affordability, and availability.

Advertisement
Are you still using workarounds to manage your daily operations? To achieve peak performance, it's time to explore other options for specialty and infusion pharmacy software. Streamline pharmacy operations and improve clinical performance with automated processing, real-time data exchange, and electronic decision support. Download this helpful infographic to: Drive efficiency and patient adherence from referral receipt to delivery and ongoing care – all with our Pharmacy Cloud.

pharmaphorum
JULY 15, 2022
In the final piece of a three-part series, Ben Hargreaves explores how manufacturing infrastructure was rapidly built up to the required capacity to ensure sufficient supply of COVID-19 vaccines, and what still remains to be done to improve vaccine equity globally. During the early stages of the current pandemic, much of the focus was taken by the possibility of developing a successful vaccine and around the R&D efforts required to achieve this.
PharmaVoice
JULY 12, 2022
RedHill Biopharma’s chief operating officer, Guy Goldberg, says the company’s oral COVID-19 therapy could still be needed on a global scale.

EMCrit Project
JULY 14, 2022
Most medical myths arose decades ago, prior to the era of modern evidence-based medicine. When investigating the origin story of those myths, we wind up reading grainy old papers from the 1950s. Surely, we think, such myths wouldn’t arise today – not in our shiny, interconnected, science-based, hyper-argumentative medical world. Unfortunately, new myths do continue […].
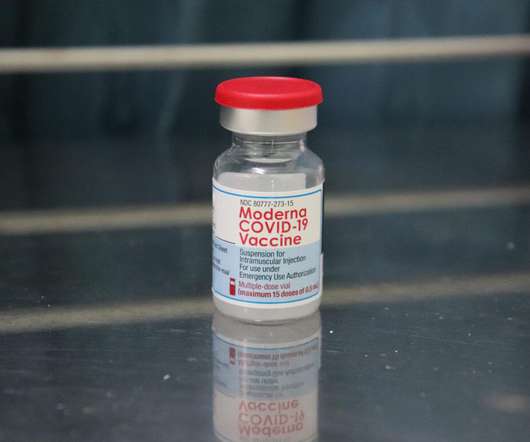
Pharmaceutical Technology
JULY 15, 2022
Health Canada has granted approval for the usage of Moderna’s messenger RNA (mRNA) Covid-19 vaccine, Spikevax, in a 25µg two-dose regimen for active immunisation to prevent Covid-19 in children aged six months to five years. So far, children aged below five years were not eligible to receive the Covid-19 vaccine in Canada. The two-dose initial vaccine regimen in children of this age group is completed in one month, which is the same dosing schedule in adults, adolescents and children aged above

pharmaphorum
JULY 12, 2022
Going beyond the pill is proving to be a win/win for patients and pharma alike – because improved outcomes are better for everyone. With growing numbers of people around the world living with long-term conditions, the pharmaceutical industry has a golden opportunity to use its expertise to truly enhance lives. But innovative new products are only part of the equation, because even the most efficacious drugs will only work as part of a holistic programme of care that includes mental health suppor

PharmaVoice
JULY 13, 2022
How the chief commercial and corporate strategy officer is embracing a ‘full-cup’ philosophy to build a better leadership toolbox.

European Pharmaceutical Review
JULY 12, 2022
According to a recent survey of patient groups, Roche, Pfizer, ViiV Healthcare and Gilead Sciences are among the top companies for implementing and reporting progress on environmental, social, governance (ESG) activities. However the survey of 1,500 patient groups also found that 55 percent were not aware of pharma’s ESG programmes, suggesting pharma needs to better include and consider the patient perspective in establishing their ESG goals.

Pharmaceutical Technology
JULY 13, 2022
Merck (MSD outside North America) and Orion have entered an international agreement to develop and market the latter’s investigational candidate ODM-208 to treat metastatic castration-resistant prostate cancer (mCRPC). Under the deal, the companies will also develop various other drugs that act on cytochrome P450 11A1 (CYP11A1), an enzyme vital for steroid production.
pharmaphorum
JULY 15, 2022
Health technology assessment (HTA) agency NICE has finalised its guidance on Amarin’s Vazkepa, clearing the path for GPs to start prescribing the drug in up to 425,000 NHS patients at high cardiovascular risk because of raised triglyceride levels. The just-published final technology appraisal document recommends that Vazkepa (icosapent ethyl) can be prescribed to people in England and Wales for adult patients with high-risk cardiovascular disease and elevated levels of triglycerides (1.7 m

PharmaVoice
JULY 11, 2022
Phil Brown, Dermavant’s chief medical officer, discusses VTAMA — the first topical cream approved for psoriasis in 25 years.

European Pharmaceutical Review
JULY 12, 2022
Microbes developing resistance to disinfectants is a major emerging problem in pharmaceutical manufacturing cleanrooms and, if left unchecked, could present a threat to drug quality and, therefore, human health. More than 480 warning letters solely related to the failure to produce aseptic conditions because of deficient cleaning and disinfection systems for cleanrooms and their equipment, were issued by regulators between 2013 to 2018.
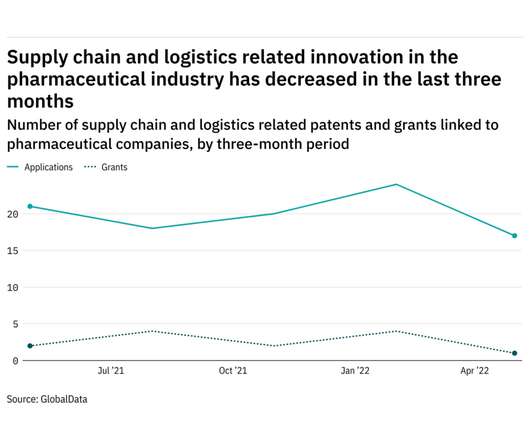
Pharmaceutical Technology
JULY 11, 2022
Research and innovation in supply chain & logistics in the pharmaceutical sector has declined in the last year. The most recent figures show that the number of supply chain and logistics related patent applications in the industry stood at 17 in the three months ending May - down from 21 over the same period in 2021. Figures for patent grants related to supply chain and logistics followed a similar pattern to filings - shrinking from 2 in the three months ending May 2021 to 1 in the same per

pharmaphorum
JULY 13, 2022
New York’s attorney general has accused Teva of lying to evade responsibility for its alleged role in fuelling the opioid epidemic in the state and is asking for a case against the company to be reopened. AG Letitia James says the Israeli drugmaker “made significant and intentional misrepresentations” to her office and the court about its involvement with its US subsidiary in order to evade legal action and accountability.

PharmaVoice
JULY 14, 2022
The company’s chief medical officer discusses its lead candidate and how it fits into wider oncology trends.
European Pharmaceutical Review
JULY 14, 2022
A new pilot project by the European Medicines Agency (EMA) will assess whether the analysis of ‘raw data’ from clinical trials improves the evaluation of marketing authorisation applications (MAAs) for new medicines as well as post-authorisation applications. The project will also evaluate the practicality of such submissions and analyses. Raw data constitutes individual patient data from clinical studies in electronic structured format that is directly accessible for analysis and visualisation.

Drug Channels
JULY 15, 2022
Today’s guest post comes from Carolyn Zele, Senior Manager of Solution Enablement at MMIT. Carolyn explains the advantages of alternative payment models (APM) for measuring and rewarding value and outcomes in our healthcare system. For more on how APMs measure value, click here to learn about MMIT’s Pulse Analytics Solution. Read on for Carolyn’s insights.

pharmaphorum
JULY 13, 2022
Roche’s Genentech unit has gone all in on its collaboration with UK biotech Bicycle Therapeutics, taking up an option on a second additional target in the area of cancer immunotherapy. Bicycle’s bicyclic peptide drug discovery platform is designed to produce drug candidates that have targeting properties like antibodies but are much smaller molecules, making manufacturing and dosing easier.

PharmaVoice
JULY 15, 2022
The incoming CEO aims to build on the buzz around the company’s leading-edge gene therapy tech.

European Pharmaceutical Review
JULY 15, 2022
The pharmaceutical sector is not typically seen as a highly polluting, ‘heavy industry’ but it is far from green. In its 2021 report Delivering a ‘Net Zero’ National Health Service , the UK’s NHS attributes as much as a quarter of its carbon footprint to medicines. A deep carbon footprint is a common hallmark of energy intensive manufacturing processes – and the manufacture of pharmaceuticals is no exception.
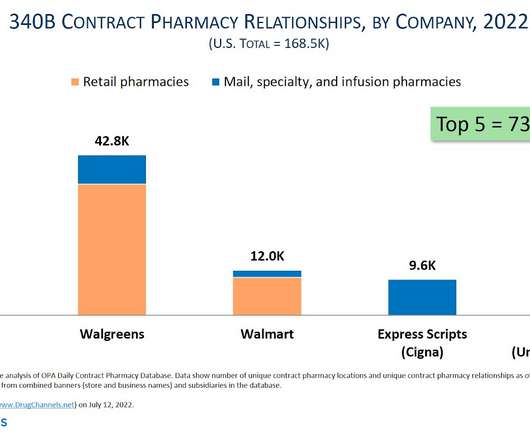
Drug Channels
JULY 12, 2022
Despite what you may have heard, pharmacy participation in the 340B Drug Pricing Program continues to thrive. Drug Channels Institute’s latest analysis reveals that an astonishing 32,000 pharmacy locations —more than half of the entire U.S. pharmacy industry—now act as contract pharmacies for the hospitals and other healthcare providers that participate in the 340B program.
pharmaphorum
JULY 11, 2022
Annie Kennedy, chief of policy, advocacy, and patient engagement at the EveryLife Foundation for Rare Diseases, tells us why the Foundation sponsored The National Economic Burden of Rare Disease Study, undertaking the challenge of examining the financial impacts of rare diseases. In 2018, the Foundation and its partners elected to perform The National Economic Burden of Rare Disease Study when it recognised that little data existed on who was living with rare diseases and what cost barriers were

Pharma Times
JULY 11, 2022
Data shows a significant reduction in treated spontaneous and traumatic bleeds among haemophilia patients
European Pharmaceutical Review
JULY 12, 2022
AstraZeneca will acquire all outstanding equity of TeneoTwo for an upfront payment of $100 million on closing. The pharmaceutical giant will make further milestone-related payments of up to $805 million related to R&D and up to $360 million on achievement of commercial milestones. The acquisition of TNB-486 further diversifies AstraZeneca’s haematology pipeline, which spans multiple therapeutic modalities and mechanisms to address a broad spectrum of blood cancers.

Drug Channels
JULY 11, 2022
Informa Connect's Hub and Specialty Pharmacy Models West. Delivered as a Hybrid Event. September 14-15, 2022 Sheraton San Diego Hotel & Marina | San Diego, CA www.informaconnect.com/hub-specialty-pharmacy-west. Drug Channels readers will save 10% off the current registration rate when they use code 22DC10 *. This important event convenes key stakeholders including manufacturers, specialty pharmacies, hub providers and more for unrivaled collaboration to leverage innovation and optimize acces
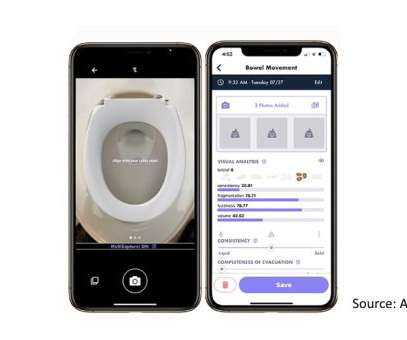
pharmaphorum
JULY 11, 2022
Researchers in the US have developed an app that uses a smartphone camera and artificial intelligence algorithms to assess images of patients’ poo for signs of disorders like irritable bowel syndrome (IBS). The tool is intended as an alternative to patients’ self-reporting stool form and frequency using the seven-point Bristol Stool Scale (BSS), which ranks consistency from hard to liquid but can produce highly variable results.
Let's personalize your content