Digital fabric could be used to measure health data
pharmaphorum
JUNE 7, 2021
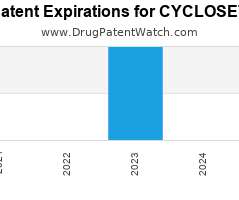
Researchers in the US have developed a wearable fabric that can sense, store and analyse data that could be used to measure physiological functions or detect illness. The team from Massachusetts Institute of Technology (MIT) claim this is the first example of a fabric which can record data in a digital rather than in an analogue format, allowing it to be programmed like any other digital device.




























Let's personalize your content